
[ad_1]
Organosulfur compounds are natural molecules that comprise a number of sulfur atoms bonded to carbon atoms. They not solely play elementary roles in organic processes but additionally have huge functions in lots of industries, corresponding to prescribed drugs, agrochemicals, and supplies science. Thus, many chemists attempt to develop protected and environment friendly strategies to synthesize organosulfurs.
The traditional strategy to supply them includes the oxidation of molecules known as thiols. Nonetheless, working with thiols will be fairly difficult. They’ve a powerful and unsightly odor and will be oxidized simply underneath air, which makes dealing with and storage tough. These two points have restricted the supply of thiols with attention-grabbing practical teams, additionally hindering the manufacturing of several types of organosulfur. However what if we may produce organosulfurs from much less problematic chemical substances?
In a latest research revealed in Natural and Biomolecular Chemistry, a analysis crew from Japan has provide you with a brand new strategy to synthesize sulfonate esters, a subclass of organosulfur compounds, utilizing thioesters. The analysis, led by Affiliate Professor Suguru Yoshida, is co-authored by Mr. Keisuke Nakamura, Ms. Yukiko Kumagai, Mr. Akihiro Kobayashi, and Ms. Minori Suzuki, all from Tokyo College of Science (TUS).
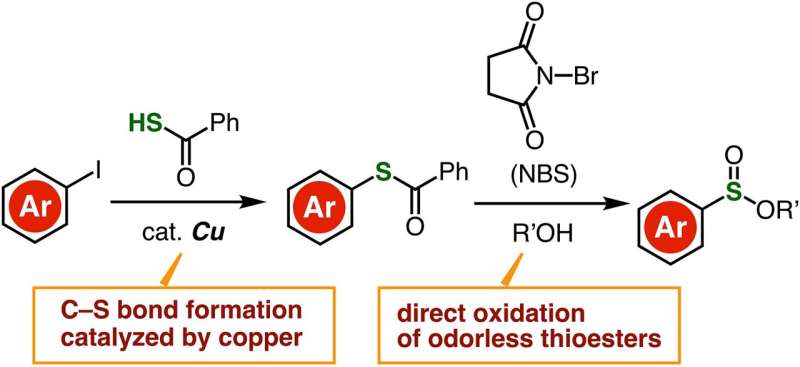
Thioesters have basically the identical chemical construction as esters, besides that one or two oxygen atoms are changed by sulfur atoms. In contrast to thiols, thioesters are odorless, steady, and simply accessible, which makes them simpler to work with. These benefits motivated the analysis crew to develop an environment friendly synthesis route for the synthesis of sulfonate esters through direct oxidation of thioesters.
They first ready a desired thioester molecule from an aryl iodide composed of an aryl group certain to an iodine atom. Utilizing a copper-containing catalyst, the researchers managed to strip the iodine atom from the aryl group and substitute it with a carbon–sulfur bond, forming a thioester. Afterwards, the thioester was immediately oxidized within the presence of N-bromosuccinimide, which created an intricate response pathway culminating with the formation of a sulfonate ester.
This two-step synthesis method is environment friendly and easy. Most significantly, it carries the potential to supply numerous sulfinate esters from simply out there beginning supplies, together with carboxylic acids, anilines, and all kinds of aryl iodides. “In comparison with typical preparation strategies of sulfinate esters from different sulfur surrogates, the superior accessibility of aryl iodides from all kinds of fragrant compounds will allow the synthesis of extremely functionalized sulfinate esters,” remarks Dr. Yoshida.
General, the tactic proposed on this research will vastly bolster analysis on new organosulfurs, resulting in promising functions in lots of fields. For instance, sulfinate esters are used within the synthesis of sulfonamide-containing compounds, which have antimicrobial, anti-inflammatory, and enzyme inhibitory actions.
They’re additionally used to supply medication with sulfoxide teams, which might have numerous organic actions, together with anti-clotting and anti-acid results. Furthermore, sulfinate esters might help synthesize practical polymers and agrochemicals and function reagents in analytical chemistry methods to detect the presence of particular compounds or practical teams.
Dr. Yoshida concludes, “Additional research in the direction of discovering functions for the preparation of bioactive organosulfur derivatives, in addition to the synthesis of bis-sulfinate esters, are underway in our laboratory.”
Extra data:
Keisuke Nakamura et al, Facile synthesis of sulfinate esters from aryl iodides through direct oxidation of thioesters, Natural & Biomolecular Chemistry (2023). DOI: 10.1039/D3OB01108A
Supplied by
Tokyo College of Science
Quotation:
Extending the taking part in subject for organosulfur: A brand new approach to synthesize sulfonate esters (2023, September 7)
retrieved 8 September 2023
from https://phys.org/information/2023-09-playing-field-organosulfur-sulfonate-esters.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.
[ad_2]