[ad_1]
In his 1870 novel “Twenty Thousand Leagues Underneath The Sea” Jules Verne describes the submarine Nautilus as powered by a sophisticated battery system, and Captain Nemo mentions that “…the cells with sodium should be considered most energetic, and that their electromotive drive is double that of the zinc cells.”1 The idea of constructing high-energy batteries proposed by Jules Verne was undoubtedly forward of its time within the late nineteenth century however consistent with the then fascination for the wonders generated by way of electrical energy. “All with electrical energy” was a dream for humanity residing within the early 1900s, however it will come into actuality within the late twentieth century with the invention of lithium-ion batteries (LIBs) based mostly on the rocking chair idea (i.e., batteries constructed with two intercalation-based electrodes with totally different potentials for storing/delivering electrochemical vitality)2,3. Presently, the worldwide manufacturing of LIBs has reached a big scale of >500-gigawatt hour (GWh), appearing as energy sources for greater than 6 million electrical autos (EVs)4. The success of LIBs testifies to the early speculation of “all with electrical energy” and opens up new avenues for extra sustainable improvement of energy-consuming anthropogenic actions.
The manufacturing capability of LIBs has risen tenfold over the previous decade5, and this demand is anticipated to proceed rising quickly over the following 10–30 years, pushed primarily by the fast-growing EV sector4. The necessity for high-performance (e.g., vitality density, security, value, and many others.) rechargeable batteries can be urgent, significantly contemplating the stringent necessities introduced by up to date sensible functions (e.g., EVs for highway and flying, drones, superior robotics, and many others.), together with inherent security and particular vitality (>500 Wh kg−1) and vitality density (>1000 Wh L−1)6. Sadly, the nonaqueous liquid electrolytes utilized in right this moment’s LIBs are unstable and extremely flammable as a result of presence of natural carbonate solvents (e.g., dimethyl carbonate, ethylene carbonate, and many others.); moreover, the graphite unfavourable electrodes with a comparatively low particular capability of 372 mAh g−1 are additionally limiting components for enhancing additional the particular vitality of the state-of-the-art LIBs6. On this regard, solid-state lithium steel batteries (SSLMBs) coupling high-energy electrode supplies (e.g., lithium steel (Li°), lithium alloys, nickel-rich LiNi1−x−yCoxMnyO2 (1−x − y > 0.8), sulfur, and many others.) with strong electrolytes are thought of a viable strategy to bypass the particular vitality density stumbling block of present LIB expertise7,8,9.
Amongst every kind of lithium-ion conductive solid-state electrolytes, strong polymer electrolytes (SPEs) have attracted vital consideration owing to their physicochemical options (e.g., excessive flexibility, ease of thin-film processing)10,11,12. Particularly, sensible high-energy functions (>1 GWh) of SPE-based SSLMBs as energy sources for EV and grid storage have been deployed by the Bolloré group since 201013. This can be a related industrial instance of SPE expertise able to offering assist for the event of high-performance SSLMBs.
For the reason that historic improvement of strong resolution electrodes in LIB applied sciences is effectively reported in latest perspective and assessment articles4,14,15,16,17, right here, we focus our consideration on the evolution of SPE supplies and their bodily chemistries, with specific reference to their functions in rechargeable secondary batteries, aiming to bridge the gaps between the earlier-developed solid-state batteries and up to date high-performance SPE-based SSLMBs. Particularly, the timeline rationale of SPE-based SSLMBs evolution is mentioned contemplating the idea of coupled and decoupled SPE methods launched by C. Austen Angell within the early Nineties18,19.
Start of solid-state batteries
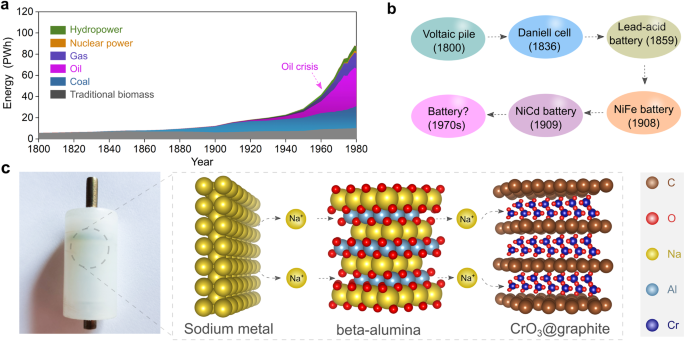
Earlier than the primary industrial revolution within the early nineteenth century, international vitality consumption relied closely on conventional biomass (e.g., wooden) and coal, as proven in Fig. 1a20. Within the Thirties, vital advances within the petroleum business enabled the shift from coal to petroleum-based high-energy sources21. Nevertheless, petroleum sources are erratically distributed worldwide, with almost half situated within the Center East. So the rising demand for petroleum-based vitality from these international locations having fewer reserves led to the oil disaster of the Seventies. This geopolitical state of affairs prompted international locations within the international north to discover different and disruptive applied sciences, in search of to rework the vitality panorama and reduce dependence on fossil fuels, significantly by means of development in high-energy rechargeable batteries22,23.
a Evolution of worldwide vitality consumption in the course of the previous two centuries. The dashed arrow notes a surge in oil consumption within the Seventies. b Transient define of batteries developed earlier than the Seventies, together with the first Voltaic pile, major Daniell cell, secondary lead-acid battery, secondary nickel–iron alkaline battery, and secondary nickel-cadmium battery27. c Photographic image of the primary solid-state cell assembled in 1972, through which sodium steel and chromium oxide intercalated into graphite (CrO3@graphite) had been utilized as unfavourable and optimistic electrode energetic supplies, respectively. The solid-state sodium-ion conductor β-alumina was adopted because the electrolyte for supporting the operation of solid-state cell at room temperature (25 °C) with reasonable stacking strain (ca. 10 MPa). The crystal buildings of sodium steel, CrO3@graphite, and β-alumina are obtained from Supplies Tasks120 and re-constructed with VESTA software program121.
Earlier than the Seventies, the particular vitality of rechargeable batteries remained decrease than 50 Wh kg−1 on the pack stage, considerably hindering their large-scale utility within the automotive business (Fig. 1b). The utilization of lithium or sodium steel (Na°) unfavourable electrodes and different high-energy electrode supplies was thought of a simple and efficient strategy to enhance the particular vitality of rechargeable batteries. Earlier than the early Seventies, a number of makes an attempt had been made to recharge lithium metal-based high-energy batteries. Nevertheless, the formation of electrically unstable lithium steel electrodeposition morphologies, equivalent to dendrites fashioned in the course of the biking course of, precipitated vital issues of safety, together with the danger of fireside and explosion24.
It thus turned clear that nonaqueous liquid electrolyte options with excessive volatility and flammability had been incompatible with lithium steel unfavourable electrodes. For utilizing high-energy steel unfavourable electrodes, a solid-state electrolyte was thought of key to constructing high-energy rechargeable batteries operated in ambient temperature area (−40 to 40 °C)25. In 1972, the very first prototype of a sodium-based solid-state cell was assembled by M. Armand25 using sodium steel because the unfavourable electrode, β-alumina because the strong electrolyte, and a chromium oxide/graphite intercalation compound (CrO3@graphite) on the optimistic electrode. The bodily picture of the cell constructed 50 years in the past is proven in Fig. 1c. Successfully, below room temperature (25 °C) and reasonable stacking strain (ca. 10 MPa), the all-solid-state Na°||CrO3@graphite cell would ship a excessive theoretical particular vitality of ca. 1000 Wh kg−1 on the materials stage (i.e., vitality content material calculated by the mass of CrO3@graphite), almost thrice larger than the nickel-cadmium batteries26.
Discovery of strong polymer electrolytes
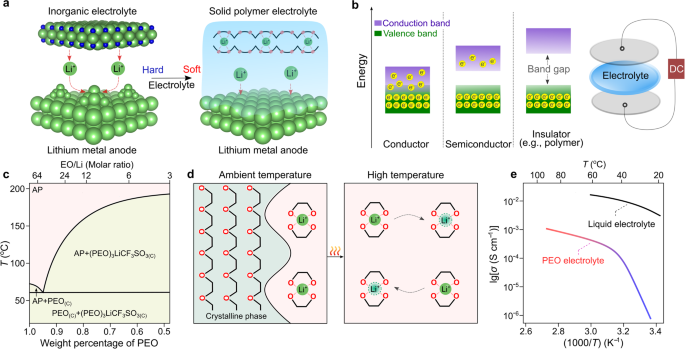
The primary convention devoted to solid-state supplies was held in Belgirate (Italy) in 197227, which significantly accelerated the event of solid-state batteries (e.g., silver steel|silver sulfide iodide|iodine cell)28,29. Along with the already identified sodium-ion conductors (e.g., β-alumina30), a number of sorts of inorganic electrolytes able to transporting lithium ions had been found earlier than the mid-Seventies, together with lithium iodide (LiI)31 and lithium nitride (Li3N)32. Nevertheless, the excessive mechanical stiffness of inorganic electrolytes (e.g., Younger’s modulus of 150 GPa for Li3N32) ends in insufficient bodily contact between the electrodes and the inorganic electrolyte, as illustrated in Fig. 2a. A excessive Younger’s modulus additionally makes inorganic electrolytes unable to accommodate the induced mechanical stresses ensuing from quantity adjustments throughout biking, leading to cracks/small gaps formation on the electrode|electrolyte interface and interphases, the evolution of parasitic aspect reactions and formation of unfavorable metallic electrodepositions (e.g., dendrites)33.
a Crucial function of sentimental contact between the electrode and a strong electrolyte. Lithium nitride (Li3N) and poly(ethylene oxide) (PEO)-based electrolytes are proven as typical examples for elucidating the distinctions between inorganic and natural supplies. The crystal buildings of Li3N are obtained from Supplies Tasks120 and re-constructed with VESTA software program121. The sunshine inexperienced, darkish blue, and pink spheres seek advice from lithium, nitrogen, and oxygen atoms, respectively; and the black sticks signify the carbon–carbon linkage between two oxygen atoms in PEO. b Electron leakage versus ionic conduction in SPEs. Polymers had been handled as insulating supplies as a consequence of their giant band gaps between valence and conduction bands (e.g., >4 eV for PEO34). The 2 discs (mild grey) on the highest and backside of the SPE membrane signify the blocking electrodes. DC: direct present. c Part diagram of lithium trifluoromethyl sulfonate (LiCF3SO3)/PEO. The values are taken from ref. 38. The sunshine inexperienced and pink areas signify the amorphous section (abbreviated as AP) area and two-phase area within the PEO-based electrolytes, respectively. PEO(C) and (PEO)3LiCF3SO3(C) denote the crystalline section of PEO and the salt/polymer advanced (i.e., (PEO)3LiCF3SO3), respectively. d Microscopic views of PEO-based SPEs at room (25 °C) and excessive (>60 °C) temperatures above the melting transition of PEO phases. e Impact of temperature on the ionic conductivity of PEO-based SPEs [(PEO)20LiCF3SO3] and standard liquid electrolyte options (e.g., 1.0 mol kg−1 lithium hexafluorophosphate (LiPF6) per kilogram propylene carbonate). The ionic conductivity values are taken from refs. 39,122.
To beat the contact points between two inflexible supplies, designing delicate strong electrolytes is an intuitive resolution. Within the late Sixties, it was established that polymeric supplies are good digital insulators (i.e., supplies with the potential of blocking the transport of electrons) with giant band gaps (>4 eV34) for electron leaping between conduction and valence bands (Fig. 2b). But, it was not clear whether or not the transportation of ionic species could be doable at the moment. In 1966, Lundberg et al.35 investigated the combination of steel salts (e.g., potassium iodide) and poly(ethylene oxide) (PEO). They concluded that steel salts work together with PEO and cut back crystallinity. In 1971, M. Armand carried out a number of ionic conductivity assessments with lithium bromide (LiBr)/PEO. From the evaluation of the outcomes, he concluded that due to the very excessive resistance (>1 MΩ) measured at room temperature (ca. 20–30 °C), the utilization of LiBr/PEO for battery functions was not really helpful. Two years later, Fenton et al.36 found that the mixtures of PEO and low-lattice-energy steel salts (e.g., sodium iodide (NaI), sodium thiocyanate (NaSCN), potassium thiocyanate (KSCN), and many others.) turn into ionically conductive upon warming up the samples (e.g., ionic conductivities for the (PEO)4KSCN advanced: 10−7 (40 °C) vs. 10−2 S cm−1 (170 °C)). This key discovering quickly caught the eye of Armand, and he prompt the utilization of those polymeric ionic conductors as strong electrolytes for constructing solid-state batteries37. These pioneering analysis works ushered a brand new course for growing delicate strong electrolytes and circumventing the floor contact concern in solid-state batteries with inorganic strong electrolytes.
Nevertheless, it was nonetheless unclear why the ionic conductivity of PEO-based SPEs was delicate to temperature. To shed some mild on this facet, Vallée et al. and Robitaille et al.38,39 systematically studied the section diagrams of a collection of lithium salt/PEO binary mixtures and revealed that PEO kinds crystalline complexes with numerous sorts of lithium salts (e.g., (PEO)3lithium trifluoromethanesulfonate (LiCF3SO3), (PEO)6lithium perchlorate (LiClO4)) and eutectic mixtures with low melting transitions from 40 to 60 °C relying on the kind of salt anions (e.g., 55 °C for (PEO)32LiCF3SO3), as proven in Fig. 2c.
Utilizing solid-state nuclear magnetic resonance spectroscopy, Berthier et al.40 demonstrated that the amorphous phases within the PEO-based SPEs are primarily chargeable for the transport of ionic species inside the SPE. Subsequently, the presence of crystalline phases at room temperature (e.g., 20–30 °C, Fig. second) was indicated as the principle purpose for the low ionic conductivities of PEO-based SPEs. Determine 2e exhibits the comparability of ionic conductivities of LiCF3SO3/PEO and lithium hexafluorophosphate (LiPF6)/propylene carbonate, that are consultant examples of SPEs, and standard nonaqueous liquid electrolyte options, respectively. Particularly, the LiCF3SO3/PEO electrolyte exhibits two totally different areas under and above the melting transition of the crystalline phases within the Arrhenius plot, which highlights the essential function of the testing temperature on the ionic conductivity of the PEO-based SPEs.
Coupled SPEs: from traditional PEO to different rising methods
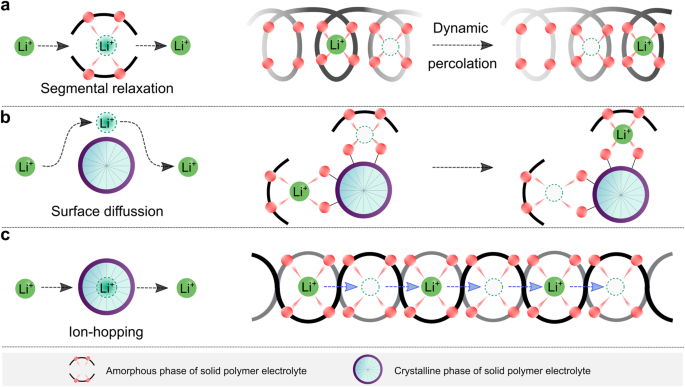
For early-developed PEO-based SPEs, the ion transport on the microscopic scale is illustrated in Fig. 3. Usually, in amorphous phases, the long-range transport of ionic species, significantly lithium ions, is especially realized by way of a segmental movement of the polymer spine (Fig. 3a), following the percolation mannequin proposed by M. A. Ratner and colleagues41. Within the combination of amorphous and crystalline phases, the crystalline floor can help ion transport by means of floor coordination (Fig. 3b)42. In distinction, the internal cores of crystalline phases don’t permit the lithium-ion transport as a result of immobilized polymer segments, like within the helical conformation of PEO within the crystalline construction of (PEO)3LiCF3SO343. It’s accepted that almost all crystalline complexes are poor ionic conductors, and the presence of those crystalline spherulites (i.e., typical morphology of crystalline PEO) in PEO-based SPEs causes a major drop in ionic conductivities by discontinuing the conduction pathways within the amorphous phases44. For a well-defined crystalline advanced (e.g., (PEO)6lithium hexafluoroarsenate), ion transport turns into doable by way of the hopping of lithium ions to adjoining websites (Fig. 3c)45. It needs to be pressured that the molecular weight of PEO and the kind of lithium salts are essential to ensure the speedy transport of lithium ions for the reason that ion-hopping pathways are strongly related to the accessible defects inside these crystalline polymers.
a Graphical illustration of the microscopic transport of lithium ions in absolutely amorphous phases, through which lithium-ion migration is tightly correlated with the segmental dynamic of polymer backbones. b Graphical illustration of the microscopic transport of lithium ions within the combination of amorphous and crystalline phases, the place the floor purposeful teams of crystalline phases favor the transport of ionic species. c Graphical illustration of the microscopic transport of lithium ions in crystalline phases, through which the cationic species migrate by way of the ion-hopping mechanism.
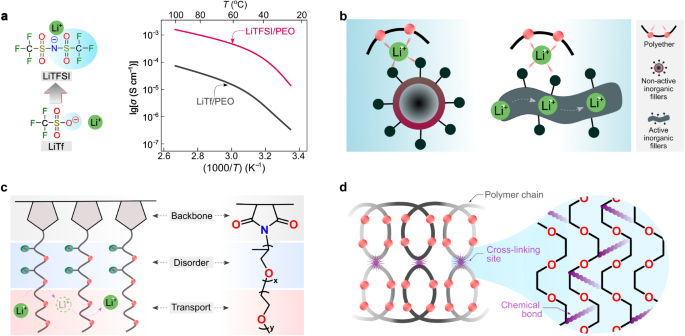
As a result of within the two former instances (Fig. 3a, b) the lithium-ion transport is related to the segmental movement of PEO, these methods are labeled as coupled SPEs. In these instances, the diploma of lithium salt dissociation and the structural flexibility (i.e., ease in conformational change) of anions decide the ionic conductivities of SPEs. Earlier than the Nineteen Eighties, the salts employed for SPEs contained primarily inorganic anions (e.g., SCN−(see ref. 36) and ClO4−(see ref. 46)), which both bind too strongly to lithium ions or have poor structural flexibility. The out there natural anions on the time, e.g., CF3SO3−, additionally bind tightly to lithium cations, as demonstrated by vibrational spectroscopic research of PEO and LiCF3SO3 mixtures47. Because of the event of sulfur-nitrogen chemistry48, in 1972, Meussdorffer et al.49 reported the preparation of the bis(trifluoromethanesulfonyl)imide (TFSI−) anion with a extremely delocalized unfavourable cost and inherent structural flexibility. This molecule was then introduced into the battery analysis subject by Armand et al.50 within the early Nineteen Eighties. As seen in Fig. 4a, changing CF3SO3− with TFSI− within the salt anion results in an order of magnitude enhance in complete ionic conductivity (i.e., the sum of cationic and anionic conductivities), reaching 10−3 S cm−1 above the melting transitions of PEO phases (e.g., ca. 2 × 10−3 at 100 °C)38. This ionic conductivity meets the minimal necessities (i.e., >10−3 S cm−1) for working SPE-based SSLMBs at elevated temperatures (≥80 °C)51,52. Within the final decade, the event of molecules with delocalized unfavourable fees has additional progressed53,54. For instance, Ma et al.54 proposed a delocalized polyanion, i.e., poly[(4-styrenesulfonyl)(trifluoromethyl(S-trifluoromethylsulfonylimino) sulfonyl)imide] (PSsTFSI−), that demonstrates improved lithium-ion conductivity of SPEs for unipolar conduction (i.e., solely optimistic fees are cell) due solely to lithium cation (e.g., at 80 °C, ca. 10−4 S cm−1 for LiPSsTFSI-based electrolyte and ca. 10−5 S cm−1 for lithium poly[(4-styrenesulfonyl)(trifluoromethanesulfonyl)imide] (LiPSTFSI)-based electrolyte54). The polyanion PSsTFSI− may very well be obtained by means of the substitute of an oxygen atom in a TFSI-like moiety (i.e., CF3SO2N(−)SO2—) with sturdy electron-withdrawing trifluoromethanesulfonylimino ( = NSO2CF3) group; thus, the unfavourable fees are additional delocalized by way of 5 oxygens and two nitrogen atoms. These analysis works exhibit an efficient technique for enhancing the ionic conductivity in coupled SPEs by weakening the interplay between salt anion and lithium ions.
a Chemical method of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and lithium trifluoromethanesulfonate (LiTf) and their ionic conductive properties in poly(ethylene oxide) (PEO)-based strong electrolytes at numerous temperatures. The values are taken from ref. 38. b Graphical illustration of the inclusion of inorganic fillers in polymer electrolytes. c Schematic illustration of amorphous Jeffamine-based polymer electrolytes. d Graphical abstract of the in situ ultraviolet photo-irradiated cross-linking technique for the PEO-based electrolytes.
Alternatively, to additional enhance the conductivity of SPEs, Croce et al.55 prompt the utilization of nano-sized inorganic fillers as energetic websites for suppressing the crystallization of ethylene oxide (EO) chains and selling the lithium-ion transport by way of floor mechanism (Fig. 4b). Inorganic fillers will be electrochemically non-active or energetic (e.g., garnet and lithium phosphorus oxynitride)56, through which the majority phases of inorganic fillers may present further transport channels apart from the polymer phases (Fig. 4b). As well as, to facilitate speedy lithium-ion transport, the morphology of inorganic fillers was expanded from nanoparticles (i.e., lower than 100 nm for all three Cartesian dimensions of the nanoparticle) to nano-wires (i.e., lower than 100 nm for the diameter of the nanowire)57,58. Functionalization of nanofiller was additionally prompt to enhance the compatibility of natural and inorganic phases59. It needs to be famous that experimental proof for the transport of lithium ions between polymer and inorganic phases will not be broadly reported, and this mechanism will not be absolutely understood. Nevertheless, it’s reported that the transport alongside inorganic bulk phases is prone to solely happen in inorganic-rich (>50 vol%) SPEs or when specific morphologies (e.g., nanowire) of inorganic phases are used56.
Varied modification methods, together with polymer blends and co-polymerization12, have been proposed within the final 20 years to suppress the crystallization of PEO matrices. For instance, the co-polymerization of EO-containing monomers with styrene-based monomers has been investigated by numerous researchers60,61,62. It has been concluded that incorporating styrene-based moieties successfully suppresses the crystallization of EO items and improves the mechanical properties of the as-formed membranes61,62. But, subtle artificial procedures are wanted to tailor the chemical buildings of the polymer matrices, which hinders the sensible deployment of such methods.
Jeffamine® compounds are industrial amine-terminated polyether oligomers produced by Huntsman Company63, broadly used as foam stabilizers and corrosion inhibitors in petroleum industries. In 1992, Benrabah et al.64 prompt the utilization of the Jeffamine moiety as a cost service area for SPEs. Systematic investigations had been additionally carried out to optimize the Jeffamine moiety’s chemical buildings. It was discovered that the Jeffamine-based comb-like polymers (i.e., polymers comprising a linear spine grafted with a number of aspect chains65) stay amorphous at room temperature (e.g., 20–30 °C) as a result of presence of structurally disordered propylene oxide (PO) items (Fig. 4c), permitting an order of magnitude enhance in ionic conductivities at room temperatures (e.g., 20–30 °C) for the corresponding SPEs in comparison with the PEO-based strong electrolytes66. Changing LiTFSI with lithium bis(fluorosulfonyl)imide (LiFSI) in Jeffamine-based electrolytes results in improved chemical and electrochemical stabilities of the lithium steel unfavourable electrode, enabling the operation of SPE-based SSLMBs near room temperatures (e.g., 20–30 °C)67.
To reinforce the mechanical energy of PEO-based SPEs, Kim et al.68 reported in situ ultraviolet photo-irradiated cross-linking of PEO-based electrolytes within the absence of risky solvents (Fig. 4d). This SPE preparation technique is also thought of for doable implementation throughout hot-pressing processes (i.e., urgent electrolyte supplies between two scorching plates) that are typical procedures for making skinny SPE movies69. The preparation of cross-linked polymers by means of different artificial strategies has additionally been reported, in addition to crystallinity suppression, ionic conductivity increment under the PEO melting temperature, and enchancment of the elastic properties of SPEs33,70. Previously many years, the structural optimizations of lithium salts and polymer matrices have improved the transport, mechanical, and interfacial properties of the coupled SPEs, endowing the SPE-based SSLMBs with secure and extremely reversible battery biking performances12.
Decoupled SPEs: outdated trend and rising methods
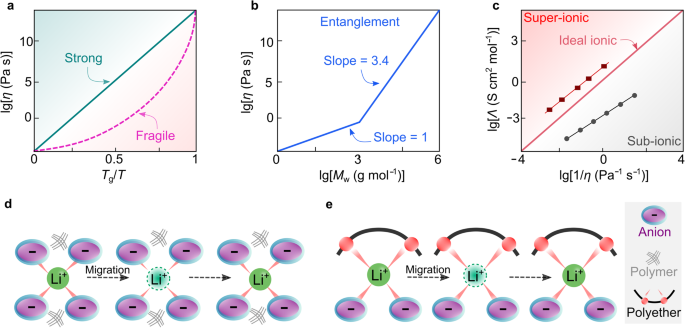
The principle shortcomings of a strongly coupled SPE system are the low ionic conductivity in response to excessive Tg and the low lithium-ion transference quantity (tLi+). Usually, the worth of tLi+ represents the portion of complete currents carried solely by lithium ions. For coupled SPEs, the lithium cations are extremely coordinated by electron-donating teams (e.g., ether oxygens) and turn into much less cell than the corresponding free anionic species (i.e., uncoordinated anions), leading to comparatively low metal-ion transference quantity (i.e., tM+ <0.5). As mentioned above, a simple strategy to rising the ionic conductivity of coupled SPE is especially by means of reducing glass transition temperatures (Tg) by way of structural manipulation. One other strategy proposed in refs. 18,71 is to search for extra fragile polymers, which will be assessed by C. Austen Angell’s plot (Fig. 5a). Right here, the time period fragile signifies a deviation from Arrhenius habits. Glass-formers with a excessive fragility are referred to as fragile, that’s, experiencing a extra speedy enhance in viscosity (i.e., sooner cooling course of) as they strategy the glass transition temperature Tg (Fig. 5a)18. On the identical temperature above Tg, the upper fragility of polymer, the bigger deviation from Arrhenius habits18,71. This ultimately results in larger ionic conductivities for the SPE comprising “fragile” polymer vs. standard polyethers. For instance, poly(vinyl chloride) (PVC) tends to be extra fragile than poly(isobutylene), and in precept, using PVC may enhance ionic conductivity. For the reason that glass transition temperature of polymer additionally strongly impacts the ionic conductivity in coupled SPEs, discovering an SPE able to concurrently satisfying the low Tg and high-fragility necessities is difficult, which limits a broader utility of this fragility idea in SPEs.
a Angell plot for sturdy and fragile strong supplies. b Entanglement mechanism of polymeric supplies. Tailored from ref. 71 with permission. Copyright 1998 Society of Chemical Business. c Walden–Angell plot for ionic supplies. The values are taken from ref. 18. d, e Schematic illustration of the microscopic ionic transport in true (d) and pseudo (e) decoupled strong polymer electrolytes. For true decoupled methods, polymers (black strains) empower the as-formed electrolytes with sure mechanical energy with out taking part within the transport of lithium ions.
Nonetheless, given the strong nature of SPEs, the increment of the polymer segmental movement alone can’t improve metal-ion diffusion sufficient until the metal-ion motions are decoupled from the polymer dynamics (e.g., segmental diffusion, chain rest, and many others). Some makes an attempt to reinforce the decoupled metal-ion movement included modifying the polymer chemistry within the spine by incorporating purposeful teams that weakly coordinate with steel ions, equivalent to poly(carbonates)72 and poly(tetrahydrofuran)73. Nevertheless, this technique has to this point didn’t considerably enhance ion decoupling and ionic conductivities. Certainly, the ionic conductivity remained low as a result of excessive rigidity of polymer backbones74.
Within the Nineties, Angell et al.75 proposed a novel strategy for attaining decoupled superionic methods (i.e., the ion transport which is decoupled from the obvious viscosity of system) by way of a so-called polymer-in-salt (PIS)-type SPEs. On this work, a excessive content material (90 wt%) of a superionic four-salt soften (i.e., LiI/lithium acetate (LiOAc)/lithium chlorate (LiClO3)/LiClO4) was combined with a small quantity (10 wt%) of high-molecular-weight (4 × 103 g mol−1) poly(propylene oxide), attaining a room temperature (e.g., 20–30 °C) ionic conductivity of about 10−4 S cm−1. The salts present ionic conductivity and the function of polymer right here is to offer mechanical energy. Based on ref. 76, the macroscopic viscosity (i.e., obvious viscosity which is experimentally measurable) of polymers will increase considerably when the numbers of repeat items exceed a threshold worth of 200, which is named the entanglement impact (Fig. 5b). Such a particular characteristic permits the solidification of this superionic liquids system upon the addition of small quantities (<5 wt%) of high-molecular-weight polymers.
The idea of decoupling in such a system is formulated on a speculation that when the salt focus retains rising to a threshold, Tg lastly will attain a most, then additional rising salt focus will improve the ion actions and will concurrently enhance the decoupling of the small steel ions from the polymer spine. Later within the early 2000s, analysis offered additional perception into the PIS electrolytes. It was proven that extra salt in SPEs results in the formation of salt aggregates through which steel ion can diffuse by means of anions. These aggregates turn into extra interconnected at excessive salt concentrations (>50 wt% of salt in SPEs)17, selling the steel ions to diffuse by means of this second conduction path. This demonstrates the flexibility of PIS-type SPEs to decouple metal-ion movement from polymer dynamics77. A number of standards, equivalent to polymer Tg, salt sort, polymer/salt solubility, electrochemical stability, and ionic conductivity78, had been additionally mentioned in C. Austen Angell’s early works to grasp the physicochemical properties of PIS electrolytes. Amongst these, the idea of the ionicity of lithium salt is of utter significance. Particularly, ionicity is a measure of the diploma of ion dissociation, generally referring to the efficient fraction of ionic species with the ability to take part in ionic conduction18. Determine 5c shows the Walden-Angell plot for the dependence of equal conductivities on the viscosities of electrolytes. With 1.0 M potassium chloride/H2O resolution as a reference electrolyte, the regime above the diagonal line refers back to the electrolyte supplies with super-ionic characters. For PIS-type SPE methods, the lithium salt ought to possess enough ionicity to make sure the excessive conductivity, i.e., be situated within the super-ionic regime in Fig. 5c.
Nevertheless, the superionic salt resolution C. Austen Angell proposed will not be relevant to lithium steel batteries as a result of poor chemical and electrochemical stabilities of the as-prepared PIS-type SPEs. Ranging from the late Nineties, a number of different PIS-type SPE methods having totally different polymer buildings and salts had been proposed and investigated, together with poly(acrylonitrile) (PAN)-in-salt79, which integrated a LiCF3SO3 salt with the PAN polymer. Sadly, this PIS electrolyte confirmed insufficient ionic conductivities, e.g., 2 × 10−6 S cm−1 at 50 °C and 10−5 S cm−1 at 75 °C79, which had been elevated to 10−4 S cm−1 at 30 °C by Wu et al.80 in 2016 used graphene oxide (GO) as a nanofiller. The authors declare that the GO nanosheets present a quick three-dimensional ion transport community. As well as, latest analysis works reported numerous advantages of utilizing PIS-type electrolytes for lithium steel batteries, together with however not restricted to secure interphase formation, improved biking efficiency81, enhanced oxidation stability82, and elevated compatibility with high-voltage optimistic electrodes83.
Nevertheless, the proposed PIS electrolytes are nonetheless removed from attaining true decoupling. Certainly, in a real decoupled system, steel ions ought to transfer independently of the polymer (Fig. 5d)18. In sensible non-ideal methods (aka. pseudo-decoupled methods), true decoupling can’t be obtained due to the chemical interactions continually occurring between ions and the repeat items (Fig. 5e). Subsequently, in a decoupled superionic system, the affect of polymers on the movement of steel ions needs to be minimized, and the ionicity of salt needs to be maximized. Experimentally, one can measure decoupled movement by evaluating time scales for polymer structural rest (i.e., cooperative reorientation of polymer segments78) with ionic conductivity rest utilizing Tg-scaled Arrhenius plot84. Thus far, the seek for superionic low melting-point salts for alkali steel ions continues to be ongoing and represents an ideal problem for the electrolyte analysis group85. Nonetheless, the technique of utilizing salt mixtures to take care of their liquid state at room temperatures (e.g., 20–30 °C) to realize excessive ionic conductivities was profitable85. Clearly, C. Austen Angell’s analysis opened a brand new avenue for designing strong and liquid electrolyte supplies86,87.
Within the subject of electrolytes, the equal conductivity of a given system is usually discovered to be inversely proportional to its viscosity, as outlined by the Walden rule (Eq. (1))88
$$varLambda eta={fixed}$$
(1)
the place Λ is equal conductivity, and η is viscosity. Successfully, a perfect decoupled electrolyte follows the Walden rule and exhibits a sure diploma of derivation from the Arrhenius habits within the ionic conductivity–temperature, supplies considerably elevated ionic conductivity as temperature elevates barely above glass transition temperature (Fig. 6a, black line). Nevertheless, as a result of entanglement impact (i.e., transient cross-links between polymer chains), the Walden rule turns into unapplicable for decoupled SPEs, and the ionic conductivities stay at a lot larger values than anticipated (i.e., viscosity doesn’t dominate the transport of the ions; Fig. 6a, purple line). Subsequently, the breakdown of the Walden rule (i.e., diminishing the influence of viscosity on ion transport) successfully favors the rise of ionic conductivity for SPEs.
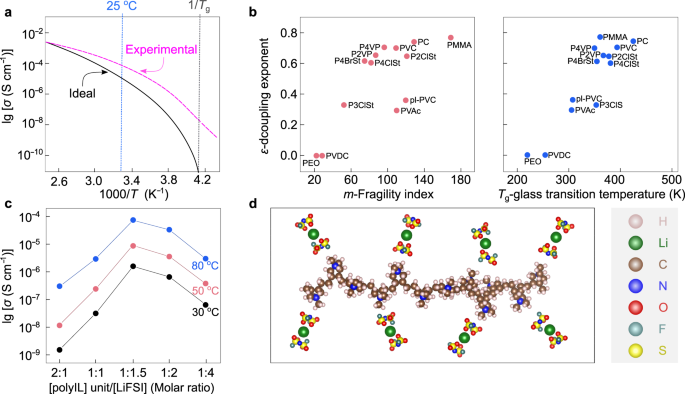
a Comparability between supreme and experimental decoupled strong polymer electrolytes (SPEs) in a typical Arrhenius plot for ionic conductivities. Tailored from ref. 71 with permission. Copyright 1998 Society of Chemical Business. b Dependence of decoupling exponent (ε) on fragility index (m) and glass transition temperature (Tg) for numerous sorts of polymers. pl-PVC plasticized poly(vinyl chloride), PC poly(carbonate), PEO poly(ethylene oxide), PMMA poly(methyl methacrylate), PVAc poly(vinyl acetate), PVC poly(vinyl chloride), PVDC poly(vinylidene chloride), P2ClSt poly(2-chlorostyrene), P2VP poly(2-vinylpyridine), P3ClSt poly(3-chlorostyrene), P4BrSt poly(4-bromostyrene), P4ClSt poly(4-chlorostyrene), P4VP poly(4-vinylpyridine). Tailored from ref. 90 with permission. Copyright 2011 American Chemical Society. c Ionic conductivities of poly(diallyldimethylammonium) (PDADMA)-based electrolytes vs. focus of lithium salts at 30 °C (black line), 50 °C (purple line), and 80 °C (blue line). Reproduced from ref. 95 with permission. Copyright 2019 Elsevier. d Interactions between lithium cations and anions within the P(DADMA+)-based electrolytes. The chemical buildings are visualized with VESTA software program122.
Agapov et al.89 systematically investigated the relations between the decoupling exponent (ε), fragility index (m), and Tg (see Fig. 6b). The decoupling exponent and fragility index are mathematically expressed under90,91:
$$mpropto left.frac{{{{{{rm{d}}}}}}[log uptau]}{{{{mbox{d}}}}frac{{{{{{mathrm{Tg}}}}}}}{{{mbox{T}}}}}proper | _{{{{{{rm{T}}}}}}={{{{{mathrm{Tg}}}}}}}$$
(2)
$$frac{{({sigma }_{{{mbox{o}}}}occasions T)}^{-1}}{tau }propto {tau }^{-upvarepsilon}$$
(3)
The place ε is the decoupling exponent, τ is the attribute rest time of the polymer section, Tg is the glass transition temperature, σo is the ionic conductivity at temperatures near Tg, and T is the temperature89. It has been demonstrated that ionic species may migrate in high-fragility polymers (fragility index m > 4089) by way of the free buildings (i.e., inflexible polymer chains with low packing density), regardless of their low segmental rest price; segmental movement is important for the less-fragile polymers with dense buildings (i.e., compact packing of versatile polymer chains), together with PEO and different polyethers.
In 2017 and 2019, Angell wrote two assessment articles to debate the analysis instructions to realize decoupling ion movement18,84, specifically, the side-chain solvation decoupling and the anion-trapping methods. The idea of side-chain solvation decoupling is to allocate the cation-solvating teams on a pendant aspect chain of polymer matrices to decouple the transport of cations from the segmental rest of the polymer spine. Sadly, the sensible implementation of this technique is difficult as a result of absence of the secondary rest impact (e.g., the movement of aspect chains) earlier than reaching enough rest time (e.g., 10−10 s) required by ion transport for battery use84. Compared, anion-trapping methods have proven some success in rising tLi+ values in polymer electrolytes both by growing polymers which might be extra susceptible to solvate anions than lithium ions (equivalent to together with under-coordinated boron facilities in polymer chains92) or designing new anion chemistries to extend its interplay with polymers and decelerate anion movement93. A extra direct technique to lure anions will be utilizing a cationic polymer electrolyte94. Nevertheless, the latest progress in growing cationic polymer electrolytes will not be solely restricted to offering anion-trapping capabilities.
For instance, Wang et al.94 reported a sort of PIS-type SPE based mostly on cationic poly(ionic liquid) (PolyIL, Fig. 6c, d), through which ionic liquid cations are chemically sure to a polymer spine, exhibiting promising physicochemical and electrochemical outcomes. This PolyIL, extra exactly poly(diallyldimethylammonium) bis(fluorosulfonyl)imide (P(DADMA+)FSI−), offered a decreased Tg at elevated LiFSI salt concentrations from a low to a excessive focus vary ([Li+]/[polycation] will increase from 0.5 to 4). The best conductivity was obtained at a excessive salt focus of [Li+]/[polycation] = 1.5 inside the temperature vary from 30 °C to 80 °C, which actually, kinds a PolyIL-in-salt (PolyIL-IS) electrolyte. Later, by means of molecular simulations, Chen et al.95 predicted the quick transport of sodium- and potassium- ions in the identical PolyIL system. Additionally they experimentally verified the sodium-ion conduction habits for the PolyIL-IS [Na+]/[polycation] = 2 system. The PolyIL-IS system demonstrated an ionic conductivity of 1 × 10−3 S cm−1 at 80 °C with out including any plasticizers (i.e., compounds for enhancing polymer dynamics), and a excessive decoupling index with log (Rτ) shut to six.3, which is obvious proof to counsel the decoupled ion movement on this PolyIL-IS96. Nevertheless, the ionic conductivities at room temperatures (20–30 °C) are nonetheless two orders of magnitudes decrease than that of standard nonaqueous electrolytes, e.g., at 30 °C, ca. 2 × 10−4 (PolyIL-IS) vs. 1 × 10−2 S cm−1 (1.0 M LiPF6/ethylene carbonate-ethyl methyl carbonate97).
Though numerous approaches have been proposed, examined, and developed, true decoupled SPEs continues to be unattainable right this moment. As C. Austen Angell talked about in his 2019 assessment article, “there’s some basic drawback within the authentic salt-in-polymer solvent (and anionic polymer) physics, as a result of ion proximity to the mobility-limiting polymer chains”84. This facet is true for PEO-type SPEs and the anionic single-ion conductors (anionic transference quantity >0.9). That is additionally a reality in most PIS-type electrolytes since solely a small portion of steel ions will be decoupled from the polymer, whereas the remainder are nonetheless sure to the polymer chains. Within the case of the PolyIL-IS methods, the weak coupling between the steel ion and the polymer exists by means of the anion-bridging co-coordination. The extremely coupled steel ion-anion movement additionally limits the metal-ion transference quantity to ca. 0.595. On this case, enhancing the ionicity of the salt may maximize the decoupling movement in PolyIL-IS, though not but experimentally proved.
SPE-based SSLMBs: from lab-scale improvement to sensible functions and future instructions
The event of SPE-based SSLMBs is carefully related to the invention of optimistic electrode energetic supplies. Lithium vanadium oxides (e.g., Li1+xV3O8, 0 <x < 2) have been lengthy investigated as optimistic electrode energetic supplies for the reason that early Seventies as a consequence of their wide selection of oxidation states enabling excessive particular capacities throughout reversible electrochemical reactions98. B. Scrosati and colleagues99 systematically studied the electrochemical performances of Li°|LiCF3SO3/PEO|Li1.2V3O8 cells and prompt that sufficient cell rechargeability may very well be attained at comparatively excessive temperatures (ca. 100 °C). With the optimization of SPEs and vanadium oxide-based optimistic electrodes, long-term (>1000 cycles) and secure biking of those SPE-SSLMBs have been achieved by the analysis group at Hydro-Québec100. But, the principle impediment to large-scale implementation of batteries with vanadium-based optimistic electrodes lies, on the cell stage, within the dissolution of vanadium species throughout steady biking, and on the uncooked materials stage, within the uneven geographical distribution of vanadium sources worldwide101.
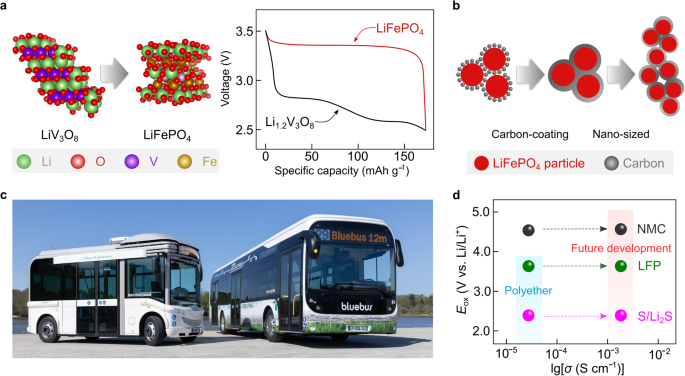
In 1997, Padhi et al.102 reported an olivine-type optimistic electrode energetic materials (Fig. 7a, left), i.e., lithium iron phosphate (LiFePO4), which exhibits a flat discharge/cost plateau at ca. 3.45 V vs. Li/Li+, and particular vitality, on the materials stage, barely larger than that of vanadium-based optimistic electrode energetic supplies (Fig. 7a, proper). Nevertheless, the digital conductivity of pristine LiFePO4 powder (e.g., 2 × 10−9 S cm−1 at 25 °C103) is decrease in comparison with different layered oxide optimistic electrode energetic supplies (e.g., 6 × 10−4 S cm−1 for lithium cobalt oxide (LiCoO2) at 25 °C104). This facet favors sluggish electrode kinetics and poor rate-capability efficiency of the LiFePO4-based batteries. In 1998, Armand et al.105 tried to extend the electrode kinetics by ball-milling LiFePO4 powder with conductive carbon in a poly(ethylene) jar. Accidentally, the ball-milling course of was left for an unexpectedly lengthy length. The optimistic electrode formulated utilizing the ball-milled LiFePO4 laminates confirmed improved enhanced rate-capabilities when examined in Li steel lab-scale cells utilizing a nonaqueous electrolyte resolution. Systematic investigations confirmed that ball-milled poly(ethylene) was transformed into amorphous carbon throughout subsequent calcination and coated the floor of LiFePO4 particles. These outcomes counsel that carbon-coating is an efficient technique to enhance the response kinetics of optimistic electrode energetic supplies with low digital conductivity (Fig. 7b). Certainly, the deployment of carbon-coated LiFePO4 considerably will increase the cycle life and attainable particular vitality of SPE-based SSLMBs105. Furthermore, the abundance of the weather in LiFePO4 suggests the next sustainability of such expertise than cobalt-based optimistic electrode energetic supplies14. The speed efficiency of LiFePO4-based SSLMBs is additional enhanced by reducing the particle sizes of LiFePO4 powders into nanoscale previous to electrode formulation (Fig. 7b)106,107.
a (left) Schematic illustration of the structural and chemical compositions of lithium vanadium oxide (LiV3O8) and lithium iron phosphate (LiFePO4, LFP). The crystal buildings of LiV3O8 and LiFePO4 are obtained from Supplies Tasks121 and re-constructed with VESTA software program122. (proper) Comparability of voltage curves of Li steel cells with SPE comprising a Li1.2V3O8– and carbon-coated-LiFePO4– based mostly optimistic electrodes cycled at 0.2 mA g−1. The values are taken from ref. 101. b Schematic representations of the LiFePO4 carbon-coating and nano-size discount methods c Photographic image of the Bluebuses® developed by Bolloré group, through which SPE-based SSLMBs are utilized as the one energy supply. The photograph is offered courtesy by the Bolloré Group. d Challenges for the up to date SPEs and their batteries, through which standard SPEs comprising polyethers are prone to be solely appropriate for low-voltage cathodes (e.g., sulfur (S), LFP), and enchancment within the electrochemical window and ionic conductivities of SPEs are desired for future improvement.
Presently, the SSLMBs comprising LiTFSI-based SPEs and LiFePO4-based optimistic electrodes have been virtually deployed as energy sources for EVs and grid storage by the Bolloré group. Determine 7c exhibits the photographic footage of Bluebuses® geared up with 120 kWh SPE-based SSLMBs. As much as 2020, greater than 1000 Bluecars® and 500 Bluebuses® have been produced, reaching a cumulated driving expertise of >6 × 108 km with a good security document (solely two instances with unexplained runaway reactions). These industrially-relevant examples stimulated industrial and tutorial laboratories to restart the analysis actions in lithium steel rechargeable batteries after the preliminary abandonment of this expertise as a consequence of the assorted hearth accidents that occurred in AA-size Li°||molybdenum disulfide cells produced by Moly Power within the late Nineteen Eighties24.
It is very important spotlight {that a} wider utility of SPE-based SSLMBs is hindered by their low particular vitality (<400 Wh kg−1) and rate-capability (< 2 C), stemming from the unsatisfactory anodic stability and ionic conductivities of the state-of-the-art SPEs (Fig. 7d). In addition to, as a result of low ionic conductivities of SPEs at room temperature (< 10−4 S cm−1 at 25 °C), the SPE-based SSLMBs should be operated at elevated temperatures (60–80 °C), thus requiring further equipment for thermal administration108. Thus far, numerous varieties of strong electrolyte applied sciences have been proposed, e.g., structurally ordered natural/inorganic composite electrolytes109, in situ fashioned polymer electrolytes110, and localized excessive‐focus electrolytes (i.e., with non-solvating diluents)111. Though these electrolyte ideas counsel believable approaches to bypass the impediment to the event of solvent-free SPE-SSLMBs, additional evaluation of the long-term stability and scalability of those applied sciences are required to maneuver towards expertise readiness ranges ≥5 (e.g., for sensible utility in large-format SSLMBs)5.
For the fundamental SPEs that merely include a single lithium salt and a single polymer matrix, it’s unclear in the event that they may very well be used to spice up the performances of SSLMBs. Though the anodic stability of the PEO-based SPEs is insufficient for coupling with high-voltage insertion-based optimistic electrode energetic supplies (e.g., nickel-rich LiNixCoyMn1−x−yO2)60, it’s doable to couple SPEs with excessive specific-energy optimistic electrode energetic supplies that exploit conversion reactions for lithium-ion storage (e.g., sulfur/organosulfur and oxygen/lithium oxide). For instance, Hu et al.112 proposed the utilization of iron fluoride (FeF3) as a conversion-type optimistic electrode to supply high-energy SPE-based SSLMBs. By introducing aluminum fluoride as an electrolyte additive, the tLi+ worth of normal PEO-based SPEs was enhanced (ca. 0.6 at 60 °C), and the corresponding Li°||FeF3 lab-scale cell confirmed excessive preliminary capacities (ca. 600 mAh g−1 at 60 °C). On this context, it must also be identified that the multi-layer construction of electrolyte movies may enhance the steadiness of the interphases fashioned on the electrode|SPE interfaces. As an illustration, utilizing a double-layer SPE comprising a polyether-based membrane for the lithium unfavourable electrode and a polyester-based membrane for the high-voltage optimistic electrode may enhance the cyclability of SSLMBs113. Furthermore, the interdiffusion of lithium salt originating from the totally different exercise coefficients (i.e., a measure for the distinction between actual and supreme options) within the two electrolytes in touch may very well be tailor-made by changing discrete anions with polyanions114.
Taking a look at future improvement, to enhance the rate-capability of SPE-based SSLMBs, it’s important to enchance the lithium-ion conductivities of SPEs by considering out-of-the-box to ship unconventional approaches for materials improvement. For instance, inheriting C. Austen Angell’s polymer-in-salt idea, wouldn’t it be doable to organize lithium salts with excessive ionicity within the liquid state at room temperatures (20–30 °C)? Ranging from LiCF3SO3, the substitute of 1 oxygen atom ( = O) with =NSO2CF3 offers LiTFSI (i.e., Li[CF3SO2(NSO2CF3)]) with a decrease melting level [i.e., Tm = 233 °C (LiTFSI) vs. >350 °C (LiCF3SO3)]115. An extra homologation of the oxygen atom ends in the formation of an excellent lithium sulfonimide salt (Li[CF3SO(NSO2CF3)2], LisTFSI) with a low melting transition approaching the ionic liquid area (i.e., Tm ≤ 100 °C for typical ionic liquids88, and Tm = 118 °C for LisTFSI116). On this regard, we speculate that the idea of unfavourable cost delocalization may very well be prolonged additional to accessing liquid lithium salts. From one other perspective, one might also change typical impartial polyether/polyesters with charged polysalts (e.g., polycations, polyanions, or poly(zwitterions)), to control the ion-ion interactions and thereby attaining decoupled SPE methods117. As an illustration, the utilization of imidazole-type poly(zwitterions) may present ordered subdomains with superionic nature, which permits speedy transport of ionic species even at temperatures near their Tg values118.
In abstract, we offered a mirrored image on SPEs and their utility in SSLMBs specializing in the important thing milestones reached during the last 5 many years of analysis and improvement. The emergence of SPEs arises from the demand for delicate strong electrolytes to bypass the contact points confronted by SSLMBs utilizing inorganic solid-state electrolytes. The utilization of SPEs stimulated the event of SSLMBs, making the long-term biking of lithium steel unfavourable electrodes doable. For future improvement, it’s important to keep in mind that designing high-performance SPE-based SSLMBs requires not solely extremely ionic conductive SPEs but additionally secure electrode|electrolyte interphases. As prompt by C. Austen Angell, decoupled SPE methods may very well be useful in designing lithium salts and polymers with tailor-made buildings and constructing lithium-ion conductive SPEs with excessive selectivity for cation transport. We advise that coupling high-energy conversion-type optimistic electrode energetic supplies with SPEs may very well be an efficient strategy to reinforce the vitality content material of the state-of-the-art SPE-based SSLMBs5, together with the advance of inherent traits (e.g., suppressed quantity adjustments, enhanced redox kinetics, excessive faucet density, and many others.) of conversion-type optimistic electrodes5. It needs to be highlighted that SPEs with enough geometric flexibility are the important thing to confronting the solid-solid contact points in SSLMBs119; and it may very well be anticipated that SPEs with improved electrochemical stabilities and ionic conductivities, significantly cation-only conductivities, will proceed to be a fascinating resolution for growing extra sustainable battery applied sciences.
[ad_2]