[ad_1]
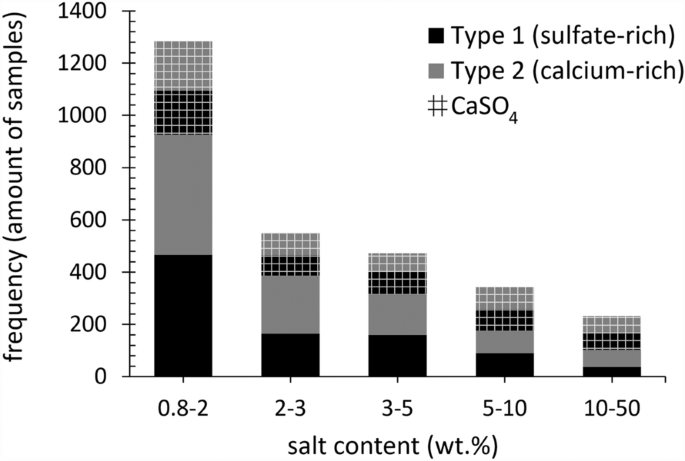
The 338 websites have been investigated for all kinds of salt-induced deterioration of porous constructing supplies. In addition to the salt combination composition, the salt focus is indicative for decay, the place enough pore filling happens, and every day environmental situations affect the substrate3,17. Thus, the primary centimeters of the drying entrance of the substrate are the first concern when evaluating the already induced deterioration, whereas the salt distribution at additional depths permits the understanding of salt transport properties over time and attainable issues for therapy strategies. The frequency of samples taken from the primary two centimeters of the substrates that embody a complete salt content material ≥ 0.8 wt.% (together with Cl−, NO3−, Na+, Ok+, Mg2+, and SO42− or Ca2+) is proven in Fig. 2, in addition to the equimolar content material of calcium and sulfate (CaSO4), thought-about because the theoretical gypsum content material (CaSO4∙2H2O), as a perform of the 2 combination kinds of curiosity.
The entire salt content material (x-axis, bin vary wt.%–wt.%) per combination sort (excl. CaSO4), with Sort 1: sulfate-rich (black) and Sort 2: calcium-rich (grey), and the CaSO4 content material per combination sort (grid) as a frequency of the samples (y-axis). The dataset is proscribed to samples with a depth from the fabric floor to 2 cm and a complete balanced ion content material ≥ 0.8 wt.% (excl. CaSO4). 1867 samples from 218 websites in complete, with 921 samples from 186 websites allotted to Sort 1 mixtures, and 946 from 132 websites to Sort 2. Excluded from the chart are 13 samples with a complete salt content material above 50 wt.%.
Within the first two centimeters of the substrate the place salt weathering to stone is usually seen, roughly 50% of the 1867 samples have a complete salt content material between 0.8 and a couple of wt.% (excl. CaSO4). Each combination sorts are nearly equally represented over the samples, with 466 and 460 allotted to combination Sort 1 (sulfate-rich) and Sort 2 (calcium-rich), respectively. The frequency of samples with salt contents above 2 wt.% declines considerably, indicating {that a} salt content material between 0.8 wt.% and a couple of wt.% is extra frequent. Gypsum is all the time current and stays comparatively comparable by way of frequency from 0.8 wt.% upwards. Nevertheless, what’s most necessary to notice is the just about equal distribution of the 2 combination sorts inside this subset, with 921 samples from 186 websites allotted to Sort 1 mixtures, and 947 from 132 websites to Sort 2 mixtures. Whereas within the full dataset (11,412 samples), thus together with all sampling depths (as detailed in33) a better frequency of as much as two thirds was noticed for Sort 1 mixtures, indicating that sulfate-rich mixtures are typically additional distributed in depth.
The evaluation of the whole dataset (11,412 samples) reveals the frequency of various ions, with not less than 5 charge-balanced ions included in > 92% of all samples, indicating the importance of mixtures against single salts. The one ion that happens much less incessantly is Mg2+, which is current in 66% of the samples. When specializing in the 2 combination sorts, Mg2+ happens in 86% of all Sort 1 and in 56% of all Sort 2 samples. Moreover, NO3− happens barely much less in all Sort 1 samples at 88%. The ion distribution inside the full dataset is additional detailed in33 and crucial ion mixtures are offered in Desk 1.
A separation of salt combination sorts, as described by Arnold and Zehnder25, with extra Sort 1 (sulfate-rich combination, together with much less soluble solids, thus much less hygroscopic) nearer to the bottom, and extra Sort 2 (calcium-rich combination, together with extra soluble solids, thus extra hygroscopic) at higher heights is probably going. Nevertheless, no statistical significance was obtained from the complete dataset as a 0.95 p-value was decided from a chi-square take a look at between the variety of samples per combination sort recognized at totally different heights: 0 to 30, 30 to 50, 50 to 100, 100 to 200, 200 to 400, and higher than 400 cm. This means that each combination sorts are attainable at any given peak. Nevertheless, each are most frequently discovered between a peak of 100 and 200 cm. This peak primarily experiences salt accumulation and decay, notably when floor water is, or was, available emigrate upwards because of the capillary forces inside the porous media.
Shifting ahead to the identification of frequent combination sorts, imply values of the ion content material for the totally different subsets and teams are used as enter information for the ECOS/RUNSALT mannequin to judge the crystallization conduct of the combination compositions. The outputs present frequent mixtures for every dataset as specified within the technique part. Comparable combination composition and conduct underneath altering RH situations are recognized, with minor variations within the variety of solids and RH ranges of curiosity per combination sort. Moreover, no important variations are seen within the solids proven within the plots between the totally different heights, depths, and supplies (brick, mortar, plaster, and stone). The imply ion values of the 2 combination sorts derived from all samples (all supplies and heights) taken from the fabric floor to a depth of most 2 cm and a complete salt content material ≥ 0.8 wt.% (excluding CaSO4) are proven in Figs. 3 and 4. The outcomes current frequent combination sorts and solids, and their conduct underneath altering relative humidity.
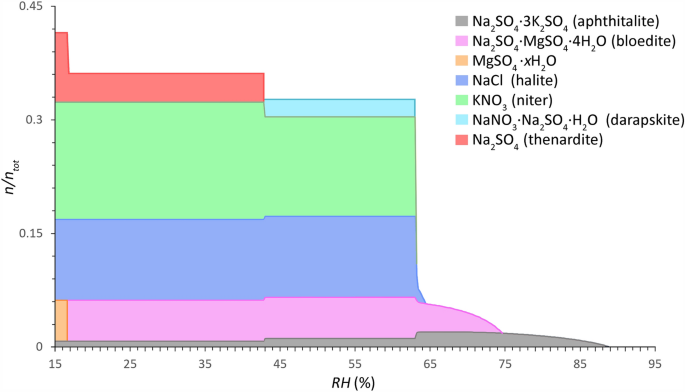
Widespread salt combination conduct of Sort 1 mixtures (sulfate-rich, much less hygroscopic) calculated between 15 and 95% RH (0.2% decision) (x-axis) at 20 °C. The relative quantity of substance is given as a fraction of crystalline salt ((n/n_{{{textual content{tot}}}})) (y-axis), imply of 921 samples from 186 websites (contemplating a most sampling depth from the floor to 2 cm and a complete salt content material ≥ 0.8 wt.% (excl. CaSO4)). Mole fractions: Cl−: 0.1070, NO3−: 0.1543, SO42−: 0.1771, Na+: 0.3063, Ok+: 0.2012, and Mg2+: 0.0540. Modified ECOS/RUNSALT output27,36.
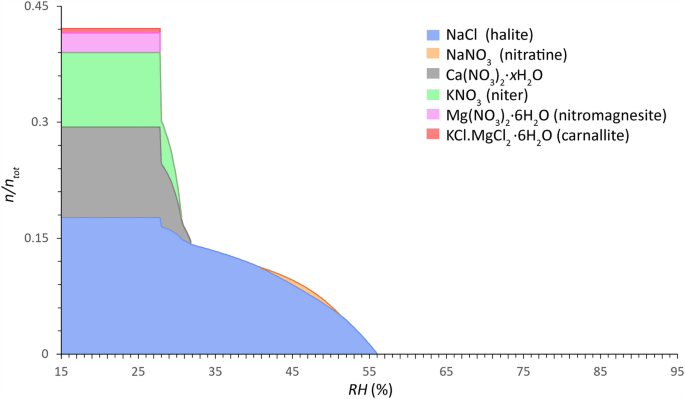
Widespread salt combination conduct of Sort 2 mixtures (calcium-rich, extra hygroscopic) calculated between 15 and 95% RH (0.2% decision) (x-axis) at 20 °C. The relative quantity of substance is given as a faction of crystalline salt ((n/n_{{{textual content{tot}}}})) (y-axis), imply of 946 samples from 132 websites (contemplating a most sampling depth from the floor to 2 cm and a complete salt content material ≥ 0.8 wt.% (excl. CaSO4)). Mole fractions: Cl−: 0.1937, NO3−: 0.3801, Na+ : 0.1770, Ok+ : 0.1016, Mg2+ : 0.0308 and Ca2+ : 0.1168. Modified ECOS/RUNSALT output27,36.
Widespread solids recognized in Sort 1 mixtures are: aphthitalite (Na2SO4∙3K2SO4), bloedite (Na2SO4∙MgSO4∙4H2O), magnesium sulfates (MgSO4∙xH2O), halite (NaCl), niter (KNO3), darapskite (NaNO3∙Na2SO4∙H2O), and thenardite (Na2SO4). Sort 2, then again, consists of halite, niter, nitratine (NaNO3), calcium nitrates (Ca(NO3)2∙xH2O), nitromagnesite (Mg(NO3)2∙6H2O), and carnallite (KCl∙MgCl2∙6H2O). Three salts happen in each combination sorts: halite, niter, and nitratine, the latter nevertheless is a part of the double salt darapskite in Sort 1. Solids which can be proven within the plots that embody magnesium can merely be eliminated with out important adjustments to the conduct when contemplating 5 ion mixtures (Cl−, NO3−, Na+, Ok+, and SO42− or Ca2+). The outputs are evaluated following the tactic described in38, with an instance output supplied and a abstract of the reactions underneath drying situations for a typical Sort 1 combination with 5 ions. From the frequent combination compositions and behaviors offered in Figs. 3 and 4 the statements made by Steiger et al.14 and Arnold and Zehnder25 are verified within the offered evaluation based mostly on the relative composition of the 2 combination sorts, with:
-
Sort 1: a sulfate-rich combination is much less hygroscopic: SO42−, Na+, Ok+, NO3−, Cl−, Mg2+
-
Sort 2: a calcium-rich combination is extra hygroscopic: NO3−, Ca2+, Cl−, Na+, Ok+, Mg2+
Particular tendencies are derived from the outputs for each combination sorts. Right here, the frequent solids and mutual crystallization RH (with a decision of 0.2%) at 20 °C for the Sort 1 combination underneath drying situations are (cf. Fig. 3): 88.8% for aphthitalite, 74.6% for bloedite, 64.4% for halite, 63.2% for niter, and 63% for darapskite. Partial decomposition of aphthitalite happens underneath drying situations under 65% and 43%, throughout step one this coincides with a rise of bloedite and crystallization of halite, niter and darapskite, adopted by a slight improve of niter and thenardite, respectively. The latter is a solid-state response and overlaps with the decomposition of darapskite. The final solid-state response happens at 17% when bloedite decomposes fully to type MgSO4∙xH2O and thenardite. Additional analysis is required to confirm the solid-state reactions and determine the magnesium sulfate hydrates, as detailed in 38.
For the Sort 2 combination, the frequent solids and mutual crystallization RH of curiosity are (cf. Fig. 4): 56% for halite, 51% for nitratine, 31.8% for Ca(NO3)2∙xH2O, 30.6% for niter, and 27.8% for each nitromagnesite and carnallite. The stable part Ca(NO3)2 was incorrectly calculated by ECOS because the anhydrous part shouldn’t be in a position to type underneath these weather conditions. The one possible type of crystallization is the tetrahydrate (nitrocalcite), nevertheless its crystallization is usually noticed to be kinetically hindered. Likewise, an unverified phenomenon is proven with nitratine because it decomposes under 41%, returning to resolution as the quantity of crystalline NaCl and the answer focus will increase.
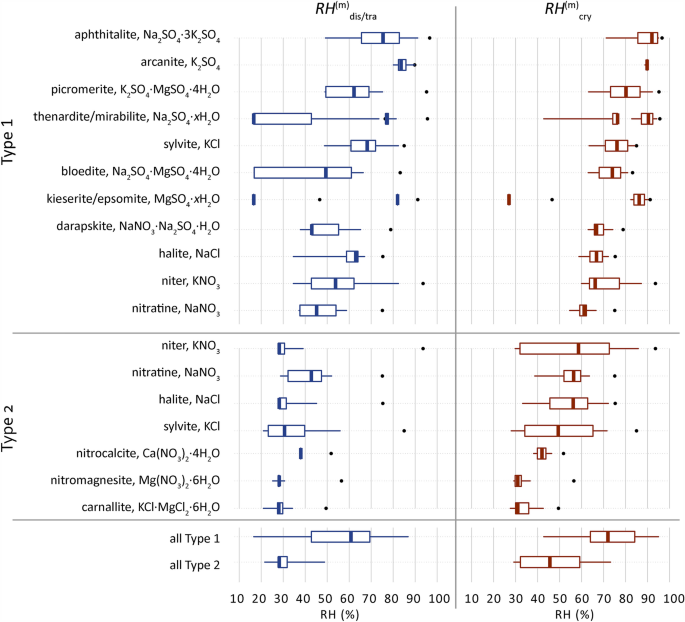
Widespread solids and their conduct are derived from the ECOS outputs of all 11,412 samples and offered in Fig. 5. The RH-range (distribution) of the stable conduct within the combination compositions are proven and grouped per combination sort. The median, minimal and most mutual crystallization, and dissolution/transition relative humidity factors ((RH_{{{textual content{cry}}}}^{{textual content{m}}} ,RH_{{{textual content{dis}}}}^{{textual content{m}}} ,RH_{{{textual content{tra}}}}^{{textual content{m}}})) are decided for every stable from all samples and in comparison with the one salt (RH_{{{textual content{cry}}}}^{{}} ,RH_{{{textual content{dis}}}}^{{}} ,RH_{{{textual content{tra}}}}^{{}}). See38 for an in depth explanations of those phrases and nomenclature.
Distribution of the mutual crystallization and dissolution or transition relative humidity factors ((RH_{{{textual content{cry}}}}^{{textual content{m}}} ,RH_{{{textual content{dis}}}}^{{textual content{m}}} ,RH_{{{textual content{tra}}}}^{{textual content{m}}})) of frequent solids recognized for Sort 1 (sulfate-rich) or Sort 2 (calcium-rich) mixtures, restricted to solids that happen in not less than 20% of samples per combination sort, right here excluding three solids per combination sort along with gypsum. Solids are recognized from the ECOS outputs of all 11,412 samples. Boxplots: 25% left, 75% proper, with whiskers drawn to the fifth and ninety fifth percentiles, excluding outliers. The dots point out the one salt (RH_{{{textual content{cry}}}}^{{}} ,RH_{{{textual content{dis}}}}^{{}} ,RH_{{{textual content{tra}}}}^{{}}). Solids with quite a lot of attainable hydrate phases are proven as xH2O and their calculated RH factors of curiosity are proven with growing hydrate from left to proper, excluding unverified metastable phases. The crystallization conduct has been calculated between 15 and 95% at 20 °C with a decision of 0.2%. Corrections are utilized following recognized points for MgSO4, Ca(NO3)2, and Ok2SO438. The 2 final boxplots (all Sort 1 and a couple of) present a abstract of all solids recognized together with ones under 20% of samples per combination sort.
Moreover, the distribution of the conduct of all solids per combination sort together with much less frequent ones (occurring in lower than 20% of samples per combination sort) are summarized to judge the general attainable conduct of the kind. The RH factors for the (single) salt are the identical in each side of Fig. 5, to make clear the (RH_{{{textual content{cry}}}}^{{}}) and (RH_{{{textual content{dis}}}}^{{}}) of the salt is similar to the RH equilibrium ((RH_{{{textual content{eq}}}}^{{}})). Whereas for solids in a mix each factors may be totally different, for instance as proven in Fig. 4, the (RH_{{{textual content{cry}}_{{{textual content{hal}}}} }}^{{textual content{m}}}) for halite is 56% and the (RH_{{{textual content{dis}}_{{{textual content{hal}}}} }}^{{textual content{m}}}) is 27.8%.
From Fig. 5, frequent solids are recognized that happen in not less than 20% of the samples per combination sort: eleven in Sort 1 and 7 in Sort 2. The RH factors of curiosity are vastly totally different in comparison with the relative humidity equilibrium for the corresponding particular person salts, with the vital crystallization RH of salts in a mix all the time under the (RH_{{{textual content{eq}}}}^{{}}) of the (single) salt. A principal RH threshold at 60% is recognized for the median mutual crystallization RH at 20 °C for all frequent solids. The solids recognized in Sort 1, excluding kieserite, present a median (RH_{{{textual content{cry}}}}^{{textual content{m}}}) above 60% RH, and for solids in Sort 2 they continue to be under this worth.
The median tendencies of all solids between the (RH_{{{textual content{dis}}}}^{{textual content{m}}}) and (RH_{{{textual content{cry}}}}^{{textual content{m}}}) are 61% and 72% for Sort 1, and 28% and 46% for Sort 2, respectively. These median values for each combination sorts are the vital RH ranges through which frequent mixtures will dissolve and crystallize, thus inflicting decay. Nevertheless, the median, most and minimal RH ranges between the (RH_{{{textual content{dis}}}}^{{textual content{m}}}) and (RH_{{{textual content{cry}}}}^{{textual content{m}}}) are additionally outlined for every stable in all of the combination compositions and point out the vary through which the stable can crystallize and dissolve. You will need to contemplate this for every particular person stable within the given combination individually, whereas protecting in thoughts that the part transitions proven for sodium and magnesium sulfate can both exist as the particular part given or a part transition can happen within the combination. The latter was not evaluated by way of frequency, thus protecting in thoughts that part transitions ((de)hydration) of a single stable can or can’t happen in a given combination, and that the one salts associated to a double salt typically coexist or type inside the given crystallization pathway.
The outcomes of the statistical evaluation present 14 frequent salts that happen in not less than 20% of samples per combination sort. The frequency of occurring solids is additional outlined in Desk 2. 5 of those solids are double salts, aphthitalite, bloedite, darapskite, and picromerite, present in Sort 1 and carnallite is incessantly recognized in Sort 2 mixtures. Much less frequent solids that aren’t included within the desk are for Sort 1: nitromagnesite (Mg(NO3)2∙6H2O) (10.2%), carnallite (KCl∙MgCl2∙6H2O) (2.6%), and bischofite (MgCl2∙6H2O) (0.6%), and for Sort 2: antarcticite (CaCl2∙6H2O) (15.8%), bischofite (MgCl2∙6H2O) (3.5%), and MgCa(NO3)4∙10H2O (2.9%). Remarking that the outcomes offered don’t embody the quantity of stable as they seem within the mixtures.
4 solids, halite (NaCl), niter (KNO3), nitratine (NaNO3), and sylvite (KCl) happen in each combination sorts. Above a frequency of 45% the solids overlap identically with the outcomes of the RUNSALT outputs of the imply ion values contemplating a most sampling depth from the floor to 2 cm, as proven in Figs. 3 and 4. Thus, concluding with the identification of the commonest solids discovered within the constructed atmosphere with seven solids recognized in Sort 1 and 6 in Sort 2 mixtures.
It stays necessary to notice that because of kinetics and attainable separation of solids from the answer throughout crystallization in real looking conditions, that’s, the in-pore scenario, deviations from the modeled crystallization pathway can happen. Moreover, the person salts of every double salt can happen, leading to a rise of the frequency relating to the solids arcanite (Ok2SO4), sodium sulfates (Na2SO4∙xH2O), magnesium sulfates (MgSO4∙xH2O), and nitratine (NaNO3) in Sort 1, and in Sort 2 mixtures sylvite (KCl) and bischofite (MgCl2∙6H2O). Moreover, the end result corresponds nicely with efflorescence detected on websites, the frequency was in comparison with reported efflorescence in 112 journal articles and convention papers41. Excluding calcium sulfate and associated double salts, the salts as efflorescence related to this examine are offered as a proportion of occasions they had been talked about in these papers: halite (49%), thenardite (45%), niter (35%), nitronatrite (32%), epsomite (30%), aphthitalite (23%), mirabilite (22%), hexahydrite (21%), syngenite (20%), darapskite (13%), nitrocalcite (11%), arcanite (7%), starkeyite (7%), picromerite (7%), bloedite (5%), and 19 others under 5%, of which kieserite, antarcticite, and pentahydrate (MgSO4∙5H2O). Naturally, extra hygroscopic salts are much less more likely to effloresce on the floor of in-situ constructing supplies.
In future analysis, it could be useful to look at the totally different combination sorts and their connections to decay phenomena noticed on-site. Sort 1 mixtures embody extra hydrating and double salts and would trigger, for instance, seen efflorescence and sub/crypto-florescence with extreme powdering, materials disintegration and/or delamination underneath common RH fluctuations round 61% and 72%. Whereas Sort 2 mixtures usually tend to lead to moisture stains, organic contamination and/or floor powdering brought on by RH fluctuations round 28% and 46%. These statements may be verified by way of on-site investigations and experimental willpower of salt combination kinetics over time, together with stable state reactions.
The kinetic facets require particular consideration towards a greater understanding of crystallization cycles underneath real looking weather conditions inside the pore construction of the fabric. Hereby specializing in pore filling, crystallization strain, adjustments within the resolution viscosity, separation of solids from the answer, pore clogging results, capillarity, crystallization pathways and climatic buffering of the fabric. Furthermore, the examine didn’t delve into the quantification of the solids recognized, which might improve our comprehension of the extent of those solids in typical combination composition. Lastly, sure hydrating salts and double salt phases want additional consideration, reminiscent of magnesium sulfates, calcium nitrates, potassium calcium nitrates, humberstonite, and calcium sulfate double salts. When evaluating the danger of fabric decay from salt mixtures and understanding the harm mechanisms over time underneath altering local weather situations, it’s essential to take all these components into consideration. This info can then be used to develop preventive measures, consider the potential for decay, and information the design of future experiments.
[ad_2]