
[ad_1]
Oxime formation from hydroxylamine and ketone: a (computational) actuality verify on stage one of many mechanism.
The mechanism of forming an oxime from nucleophilic addition of a hydroxylamine to a ketone is taught early on in most programs of natural chemistry. Right here I topic step one of this response to type a tetrahedral intermediate to quantum mechanical scrutiny.
- The primary determination is to resolve which atom of the hydroxylamine acts because the nucleophile. Response 1 exhibits the oxygen and response 2 the nitrogen. The textual content books will let you know that nitrogen nucleophiles are higher than oxygen ones. It is because nitrogen is much less electronegative than oxygen (it has a smaller nuclear cost) and so binds its single lone pair much less tightly than oxygen does its two lone pairs. Typically the Klopman-Salem equation is invoked, which tells you that the reactivity is straight proportional to the overlap between the donor MO and the (empty) acceptor MO, and inversely proportional to the vitality hole between these two orbitals. Nitrogen wins out as a result of its lone pair is “bigger” and therefore overlaps higher, and since its donor MO vitality is larger than oxygen and therefore the vitality hole between it and the π* C=O acceptor is decrease.
- Actuality verify: We have to assemble an appropriate transition state for each prospects, after which examine their free energies. There’s a alternative of selecting a stepwise pathway (the one proven in all of the textual content books) wherein the bond from N or O to C is fashioned in an preliminary step, after which adopted by a step usually simply labelled PT to switch the proton utilizing solvent molecules. These two steps will also be conflated right into a single concerted mechanism involving a six-membered ring transition state. Quantum mechanically, this latter choice has the benefit of avoiding any nice construct up of cost separation at any stage within the mechanism, however has the drawback that the entropic loss on the transition state is larger (though “borrowing” a water molecule from a bulk solvent for this objective is less complicated than doing so from an infinite distance away).
- Proven under is a ωB97XD/6-311G(d,p)/SCRF=water calculation of the transition state for N-attack. It has a dipole second of 6.2D, which is actually fairly small, and much from that anticipated for the zwitterionic intermediate proven within the stepwise mechanism (that might be between 15-30D).
Cyclic transition state for N-attack.
- The intrinsic response coordinate exhibits a concerted response with fairly a small barrier. It’s small as a result of the nitrogen is the truth is a super-nucleophile, its nucleophilicity has been augmented over that of a easy amine by a so-called α-effect from the adjoining two pairs of lone pairs on the oxygen activating the nitrogen lone pair by lone-pair repulsions.
- The gradient norm alongside the coordinate additionally exhibits an virtually synchronous response. The one blip happens at round IRC +1.3, and this corresponds to the switch of a proton from NH to a water molecule. An earlier proton switch from water to the carbonyl oxygen was basically synchronous with formation of the N-C bond. This synchronicity is what helps keep away from any giant construct up of cost separation. For that reason, I can’t assist however really feel that the textual content books may take in this lesson and present a cyclic concerted response mechanism as a possible various to 2 stepwise processes.
- Proven under is a ωB97XD/6-311G(d,p)/SCRF=water calculation of the transition state for N-attack. It has a dipole second of 6.2D, which is actually fairly small, and much from that anticipated for the zwitterionic intermediate proven within the stepwise mechanism (that might be between 15-30D).
- Subsequent, O-attack. The IRC for this isomeric mode exhibits a considerably larger barrier in comparison with N (the computed relative free energies show the O to be larger by 8.3 kcal/mol than the N) and smaller exothermicity. It reveals even better synchrony of the 2 proton transfers with the O-C bond formation. So we have now a actuality verify of the text-books on this level within the type of an vitality distinction, which is all the time helpful.
- Now that our proton transfers are concerned within the mechanism, it’s time to take a better take a look at the geometry of those transfers. On this level, the textual content books inform us that essentially the most beneficial geometry for a proton switch is having the proton co-linear with the 2 oxygens. While that is largely true for the geometries proven above, the ensuing six-membered ring consequently adopts a triangular form, which isn’t preferrred for the bond angles. This could possibly be solved by incorporating a second water molecule, to present us mannequin 3 proven above.
- A second water molecule may be positioned in two various positions. The primary merely solvates the 6-ring transition state. The second actively participates by way of an enlarged eight-membered ring transition state. It seems that the latter is decrease by 4.5 kcal/mol in free vitality, largely as a result of much better bond angles and the virtually precisely linear proton transfers now potential.
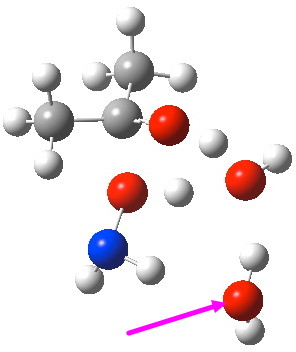
O-Transition state with two water molecules, one merely hydrogen bonding to the 6-ring (magenta arrow).
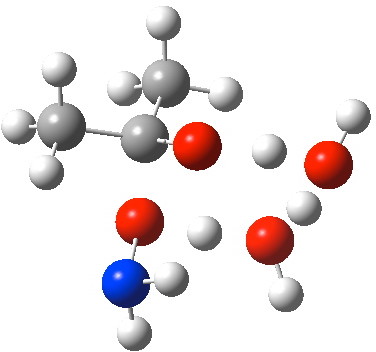
O-Transition state with two water molecules, each a part of a cyclic transition state.
- So the next is our greatest mannequin. It’s 10.4 kcal/mol decrease in free vitality than the isomeric O-attack transition state. The timing of the bonds exhibits that N-C formation coincides with the primary proton switch to the carbonyl oxygen, adopted by an O to O proton switch and eventually N to O. The dipole second on the transition state is 5.9D, revealing little specific cost separation.
N-attack by way of an 8-ring transition state.
- A second water molecule may be positioned in two various positions. The primary merely solvates the 6-ring transition state. The second actively participates by way of an enlarged eight-membered ring transition state. It seems that the latter is decrease by 4.5 kcal/mol in free vitality, largely as a result of much better bond angles and the virtually precisely linear proton transfers now potential.
It’s price concluding this exploration by reiterating that the fashions above are usually not full. A bulk solvent would enable (statistical) participation of extra than simply two solvent molecules, and the dynamics of such a (very complicated) course of has but to be explored. However I hope what you see here’s a bit nearer to “actuality” than many a text-book writer has after they illustrate their books.
doi:10042/a3uxl
Associated
Tags: animation, vitality distinction, free vitality, Fascinating chemistry, nucleophilic assault, Response Mechanism, text-book writer, Tutorial materials
You possibly can go away a response, or trackback from your personal web site.
[ad_2]