[ad_1]
The properties of the aqueous densified electrolyte
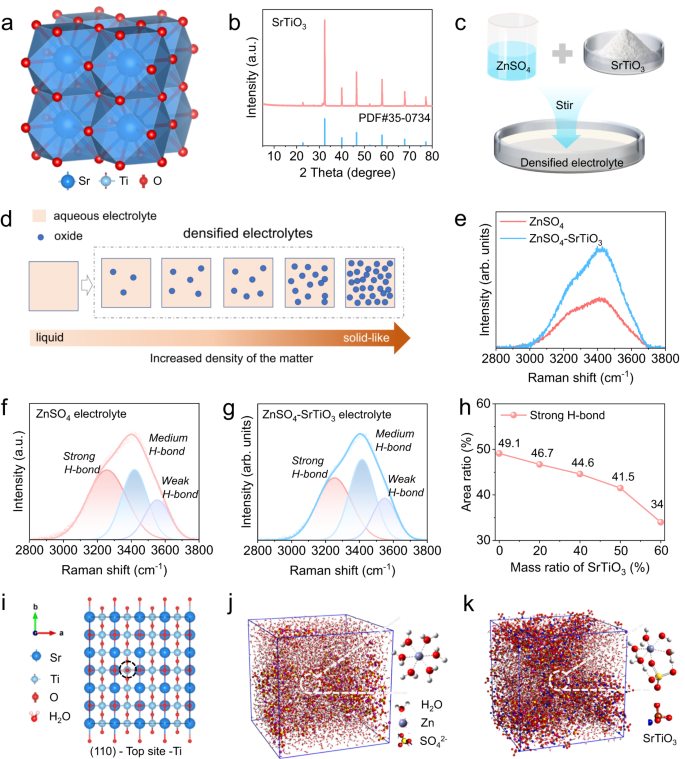
Determine 1a illustrates that SrTiO3 is a cubic perovskite construction, crystallizing within the cubic Pm-3m area group. Sr2+ is bonded to 12 equal O2− atoms to type SrO12 cuboctahedra, sharing corners with twelve equal SrO12 cuboctahedra and sharing faces with six equal SrO12 cuboctahedra and eight equal TiO6 octahedra33. The XRD results of the SrTiO3 powder used to supply the densified electrolyte exhibits that the powder has a extremely pure crystal construction (Fig. 1b). The particle sizes of those powders are lower than 5 μm, with comparatively uniform particles and good dispersion, as noticed by scanning electron microscope photos (Fig. S1). Specifically, the aqueous densified electrolyte is ready by mixing 2 M ZnSO4 resolution and SrTiO3 powder in a mass ratio of one-to-one, after a typical mechanical stirring (Fig. 1c). It’s value mentioning that densified electrolytes could be composed of various aqueous electrolytes and varied oxides, usually referring to the electrolyte with elevated density after the addition of oxides (Fig. 1d). The produced densified electrolyte is a grayish-white homogeneous dispersion liquid of SrTiO3 particles, which has some solid-like or non-Newtonian fluid options (Supplementary Fig. S2). The conductivity of the densified electrolytes is barely smaller than that of the standard electrolyte, and progressively decreases with the rise of SrTiO3 content material, which is especially as a result of SrTiO3 is an insulating materials and the viscosity of the densified electrolyte is larger than that of the standard electrolyte (Supplementary Fig. S3). To discover the impact of SrTiO3 particles on electrolytes, Raman spectroscopy was used to detect adjustments within the state of water molecules within the densified electrolyte. In typical electrolytes, specifically 2 M ZnSO4 resolution, a small a part of water molecules is solvated by Zn2+, and many of the remaining free H2O molecules type a water community by intermolecular hydrogen bonding forces34. In contrast with the standard electrolyte, the Raman spectra of the densified electrolyte change considerably (Fig. 1e), indicating that the state of water molecules adjustments accordingly below the affect of SrTiO3 particles. For additional investigation, an in depth quantitative evaluation of Raman spectra was carried out. Inside the vary of 2800–3800 cm−1 in Raman spectra, peaks are derived from O-H vibrations. To be exact, the height within the high-frequency area round 3550 cm−1 is the low power O-H in H2O, that’s, the weak hydrogen bond. The height within the low-frequency area of 3253 cm−1 is the high-energy O-H in water, similar to the robust H-bond. The height close to 3416 cm−1 is attributable to the medium H-bond35. Within the typical electrolyte, the robust H-bond has the very best peak power and the widest peak space of 49% (Fig. 1f), suggesting the excessive reactivity of the free H2O molecules. In sharp distinction, the medium H-bond has the very best peak power, and the world of the robust hydrogen bond is lowered to 41% within the densified electrolyte (Fig. 1g), which signifies that SrTiO3 particles destroy the H-bond community construction and weaken the reactivity of free water within the densified electrolyte. To additional examine the variation of the H-bond community construction with SrTiO3 content material, Raman spectra of electrolytes with completely different SrTiO3 contents (0 ~ 60 wt%) and their corresponding becoming peaks have been analyzed. As proven in Fig. 1h, the robust H-bond situated at 3253 cm−1 reveals a transparent downward pattern because the content material will increase. When the content material is as much as 60 wt%, the proportion of robust H-bond is just 34%, which signifies that extra SrTiO3 particles can harm extra H-bond community of the electrolyte (Supplementary Fig. S4). Within the densified electrolyte, SrTiO3 particles have an excellent means to adsorb water molecules based on DFT calculation (Supplementary Fig. S5). The adsorption power of H2O on varied geometrical configurations demonstrates that the binding power of H2O adsorbed on the Ti atom of SrTiO3 (110) airplane is as much as −1.02 eV, which is considerably larger than that of different websites (Supplementary Fig. S6), indicating that this web site has the perfect water molecular affinity (Fig. 1i). For additional research of the solvated H2O molecules, molecular dynamics simulations (MSD) have been used to review the solvated construction of Zn2+. In response to the calculation outcomes, the standard solvation construction in a traditional electrolyte is that six H2O molecules are solvated by one zinc ion, specifically Zn(H2O)62+ (Fig. 1j). Due to this fact, SrTiO3 particles adsorbs water molecules, leading to a change within the solvation construction of Zn2+, the commonest construction is the SO42− into the solvation shell (Fig. 1k). The calculated radial distribution features and the corresponding integrals of Zn-O(H2O) and Zn-O(SO42−) demonstrates that the discount of solvated water molecules within the densified electrolyte (Supplementary Fig. S7). Combining the experimental and computational outcomes, it may be concluded that the addition of SrTiO3 particles can’t solely change the bodily properties but in addition change the state of each free and solvated H2O molecules in a densified electrolyte.
a The crystal construction of SrTiO3. b The XRD spectra of the SrTiO3 powder and its customary PDF card. c Schematic diagram of densified aqueous electrolytes. d Schematic illustration of densified electrolytes shaped by growing density of resolution after the addition of oxide. e Comparability of Raman spectra of various electrolytes. Raman match peaks of (f) ZnSO4 electrolyte and (g) aqueous densified electrolyte. h The ratio of becoming robust H-bond space of electrolytes with varied SrTiO3 contents. i The Prime view of geometrical configurations of H2O adsorbed on the Ti atom of SrTiO3 (110) airplane. The containers of molecular dynamics simulations with fundamental solvated construction of Zn2+ in (j) ZnSO4 electrolyte and (okay) aqueous densified electrolyte.
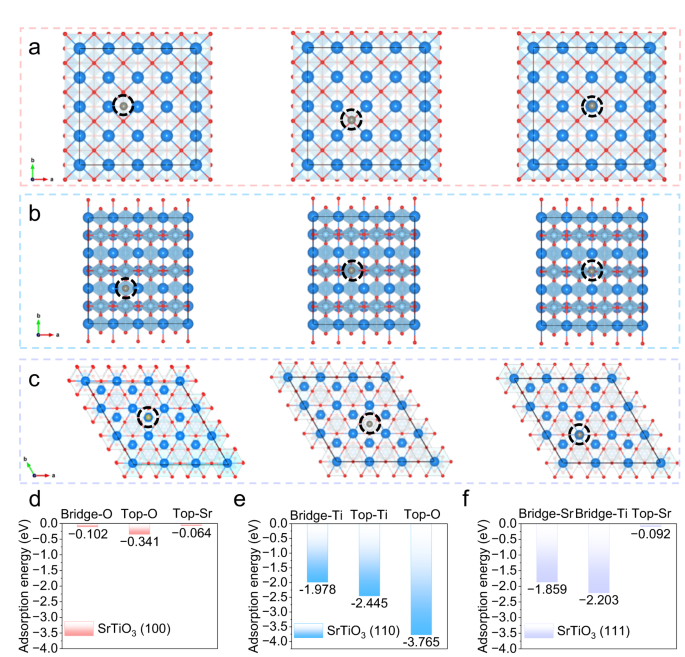
To deeply examine the interplay between SrTiO3 and zinc atoms on the micro-level, theoretical calculations based mostly on DFT have been carried out. The crystal planes of (100), (110), and (111) of SrTiO3 have been chosen as analysis objects, and two varieties of adsorption websites, bridge, and prime, have been thought-about in every crystal airplane. Detailed prime views of the geometrical configurations of zinc atoms absorbed on the (100) airplane of SrTiO3 are displayed in Fig. 2a, and the corresponding adsorption power is proven in Fig. 2nd. On this airplane, the adsorption power of zinc atoms on the bridge-O, top-O, and top-Sr websites may be very low, which signifies that the affinity of (100) airplane of SrTiO3 with zinc atoms is poor. Whereas the (110) airplane reveals the strongest adsorption power to zinc atoms, amongst which the highest oxygen web site reveals the very best adsorption power of −3.765 eV, indicating that the (110) airplane has the perfect affinity for zinc atoms (Fig. 2b, e). The adsorption power of the (111) airplane offers the very best adsorption power of two.203 eV on the bridge-Ti web site, which is considerably larger than that of the (100) airplane, however barely decrease than that of the (110) airplane (Fig. 2c, f). The above outcomes present that the (110) airplane has the perfect affinity for zinc atoms. Extra importantly, based on the XRD sample of SrTiO3 powder, the (110) crystal face has the strongest peak, specifically essentially the most uncovered crystal face below the pure situation, demonstrating that the aqueous electrolyte densified by the addition of SrTiO3 offers glorious affinity for zinc atoms.
Prime view of geometrical configurations of zinc atoms absorbed on SrTiO3 planes (the darkish blue ball represents the Sr atom, the indigo ball is the Ti atom, the pink ball is the O atom, and the grey ball is the Zn atom): a (100) airplane, b (110) airplane and c (111) airplane. The corresponding adsorption power between zinc atoms and varied adsorption websites on SrTiO3: d (100) airplane, e (110) airplane and f (111) airplane.
The advantages within the aqueous densified electrolyte
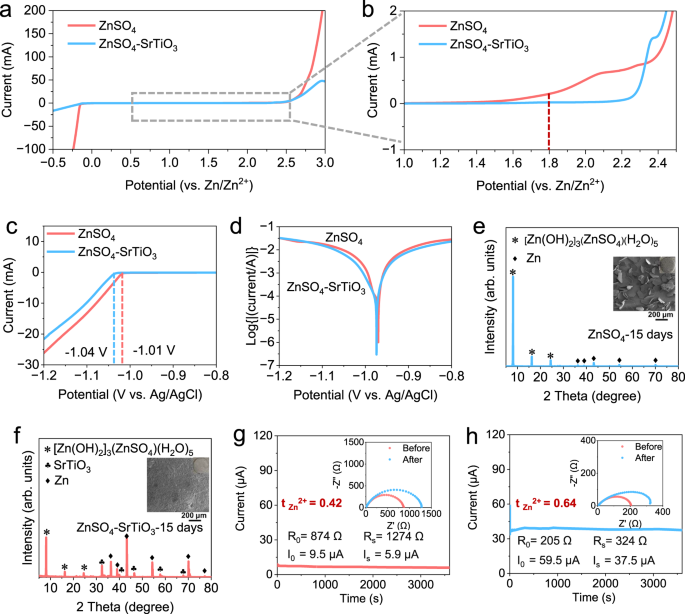
To research the potential software of densified electrolytes in zinc-ion batteries, a sequence of electrochemical characterizations have been carried out. The electrochemical stability window between 2 M ZnSO4 and densified electrolyte was studied by linear sweep voltammetry (LSV) checks at a scan price of 10 mV s−1 on coin cells utilizing zinc metallic as reference and counter electrodes, and stainless steels as working electrodes. Inside the vary from −0.5 to three.0 V, there is no such thing as a apparent distinction within the decomposition potential of the 2 electrolytes, however there’s a massive distinction within the response present (Fig. 3a). The present for hydrogen evolution response (HER) in 2 M ZnSO4 reaches as much as 100 mA at −0.25 V, in sharp distinction, the present for the densified electrolyte is beneath 20 mA at −0.5 V. When the curve is magnified, the onset potential of the oxygen evolution response (OER) is 1.8 V for typical electrolyte, whereas that of the densified electrolyte is above 2.2 V, confirming that SrTiO3 particles improve the electrochemical stability of densified electrolyte (Fig. 3b). The HER have been additional investigated by a three-electrode system with 30 mV discount within the onset potential of the densified electrolyte (Fig. 3c). Aspect reactions between zinc metallic anodes and electrolytes are the primary issue of Coulombic effectivity discount and cells failure36. The Tafel plots present that the densified electrolyte reveals a decrease corrosion present density, suggesting that SrTiO3 can inhibit facet reactions and alleviate the corrosion price of Zn anodes (Fig. 3d). To additional examine the by-products attributable to facet reactions, a soaking experiment with zinc foils immersed within the electrolytes was proposed. When the Zn foil was immersed in 2 M ZnSO4 for 15 days, there are seen flaky by-products on the floor (Supplementary Fig. S8a). The SEM picture exhibits that the form of the by-products on the floor of the immersed zinc foil are irregular polygons with common lengths of greater than 100 μm. And the corresponding XRD sample demonstrates very robust peaks of [Zn(OH)2]3(ZnSO4)(H2O)5 (ZSH), even masking the peaks of Zn, suggesting that critical facet reactions have occurred on the floor (Fig. 3e). As a distinction, the zinc foil immersed within the densified electrolyte has a skinny off-white layer on its floor as a result of SrTiO3 is tough to adequately wash off (Supplementary Fig. S8b). The zinc foil in densified electrolyte exhibits a easy floor with none massive flake. The XRD sample agrees properly with the morphology remark and exhibits that there are residual SrTiO3 particles and a small variety of by-products on the floor (Fig. 3f). Mixed Tafel plots and soaking experiments, facet reactions between densified electrolytes and zinc foils considerably inhibited, which is attributed to the absorption of each solvated and free water molecules to SrTiO3 particles within the densified electrolyte, based on the outcomes of Raman spectra and MSD outcomes.
a Electrochemical stability window. b Corresponding zoomed-in curves of tiny areas. c The linear sweep voltammetry (LSV) for check of HER. d Tafel curves in three-electrode cells. XRD patterns of Zn foils (the insets are the corresponding SEM photos) soaked in (e) ZnSO4 electrolyte and (f) the densified electrolyte. The Zn2+ transference quantity measured by electrochemical polarization (the insets are related EIS earlier than and after the polarization of cells) at a small voltage of 10 mV in (g) ZnSO4 electrolyte and (h) the densified electrolyte.
An essential however simply missed electrolyte parameter is the transference quantity. The Zn2+ transference quantity is outlined because the fraction of the overall present carried by Zn2+ and displays the electromobility of Zn2+. In response to the Sand’s time37, a low Zn2+ transference quantity would lead to a lower within the efficient ionic conductivity, and the elevated focus polarization, additional results in the expansion of zinc dendrites. The equations calculated the Zn2+ transference quantity as follows38,39:
$${{{{t}}}_{{{{{{rm{Zn}}}}}}}}^{2+}={{{I}}}_{{{{{{rm{S}}}}}}}(Delta {{{V}}-{{I}}}_{0}{{{R}}}_{0})/{{{I}}}_{0}(Delta {{{V}}-{{I}}}_{{{{{{rm{S}}}}}}}{{{R}}}_{{{{{{rm{S}}}}}}})$$
(1)
the place R0 is the resistance earlier than polarization, RS is resistance after polarization, ΔV is the polarization potential, I0 is the preliminary present, and IS is the secure state present. The symmetric cell utilizing 2 M ZnSO4 electrolyte delivers a low worth of 0.42 (Fig. 3g). The transference numbers are considerably elevated by including SrTiO3, and the obtained quantity will increase to 0.64 with a big decreased cost switch resistance (Fig. 3h). These outcomes point out that the densified electrolyte has a distinguished efficiency in broadening the electrochemical window, selling the interface stability, and bettering Zn2+ transference quantity.
The deposition habits of zinc ions in aqueous densified electrolyte
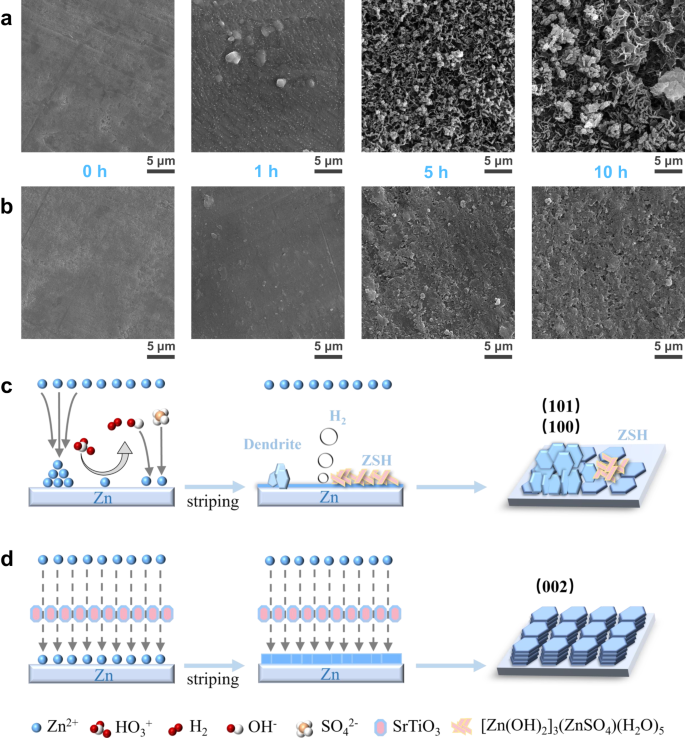
To research the influences of SrTiO3 particles on zinc deposition, the morphologies of deposited Zn anodes at a hard and fast present density of fifty μA cm−2 within the two completely different electrolytes have been examined by SEM. The evolution of zinc deposition in 2 M ZnSO4 is proven in Fig. 4a. With the deposition time elevated from 1 to 10 h, zinc dendrites develop wildly and are accompanied by a lot of by-products on the floor of Zn foils. Throughout the first hour of zinc deposition in typical electrolytes, though solely a small quantity of zinc was deposited, uneven bulk accumulation had been noticed. When the deposition time was elevated to five h, the uneven zinc deposition was aggravated, with some areas inflicting dendrites because of extreme deposition whereas the remaining areas inflicting vacant websites because of lack of zinc deposition. When the deposition capability reaches 500 μAh cm−2, along with the apparent dendrites, massive accumulations of by-products are additionally noticed on the floor of zinc foil. In sharp distinction, the zinc foil deposited within the densified electrolyte reveals a flat deposition habits, as proven in Fig. 4b. The zinc nucleation was uniform and no bulk aggregation could be noticed throughout the first hour of zinc deposition. When the zinc deposition time reaches 5 h, a flat floor freed from dendrites and by-products could be clearly noticed. The deposited zinc shaped a smoother and denser floor with none by-products because the deposition capability reaches 500 μAh cm−2. Extra importantly, the XRD outcomes of zinc foils after 10 h deposition present that the preferential crystal planes of zinc deposition change considerably in two electrolytes. The XRD sample reveals that the ratio of the height depth of Zn (002) airplane to Zn (100) airplane (IZn(002)/IZn(100)) is 1.8, and there’s an apparent peak of the by-product of ZSH in typical electrolyte. The worth of IZn(002)/IZn(100) considerably will increase to 2.9 within the densified electrolyte, suggesting that there’s a massive preferential development of Zn (002) airplane within the densified electrolyte (Supplementary Fig. S9). This may be attributed to the excessive affinity of SrTiO3 for zinc atoms, resulting in the impact of uniform and constant deposition of zinc even from the early stage of nucleation.
SEM photos of Zn foil floor as a perform of time for Zn deposition at a hard and fast present density of fifty μA cm−2. a Within the typical electrolyte. b Within the densified electrolyte. Schematic diagram of the zinc deposition processes in several electrolytes: c the standard electrolyte and d the densified electrolyte.
Mixed with the SEM photos and XRD patterns, the deposition habits of zinc ions within the typical electrolyte is clearly completely different from that in densified electrolytes. As proven in Fig. 4c, the heterogeneous deposition of zinc ions because of the non-uniformity of the electrical discipline and the focus discipline results in the expansion of zinc dendrites in typical electrolytes. Worse nonetheless, water molecules with excessive reactivity are lowered on the floor to supply hydrogen, which won’t solely result in fuel bloating but in addition trigger the rise of native pH because of the manufacturing of OH−. As soon as OH− ions are involved with zinc ions and SO42−, ZSH will quickly produce and additional result in the corrosion and passivation of the Zn floor. These horrible conditions take a dramatic flip in densified electrolytes, as proven in Fig. 4d. On the one hand, the facet reactions between the interfaces are considerably inhibited because of the weakened exercise of water molecules within the densified electrolyte. However, the SrTiO3 particles have an excellent zinc ion affinity, which may successfully induce the deposition of zinc ions alongside the Zn (002) airplane. When Zinc ions are deposited preferentially alongside Zn (002) airplane, the deposited Zn flakes are inclined to develop at a smaller angle (~0 – 30° to the substrate), reaching uniform Zn deposition and suppression of Zn dendrites26.
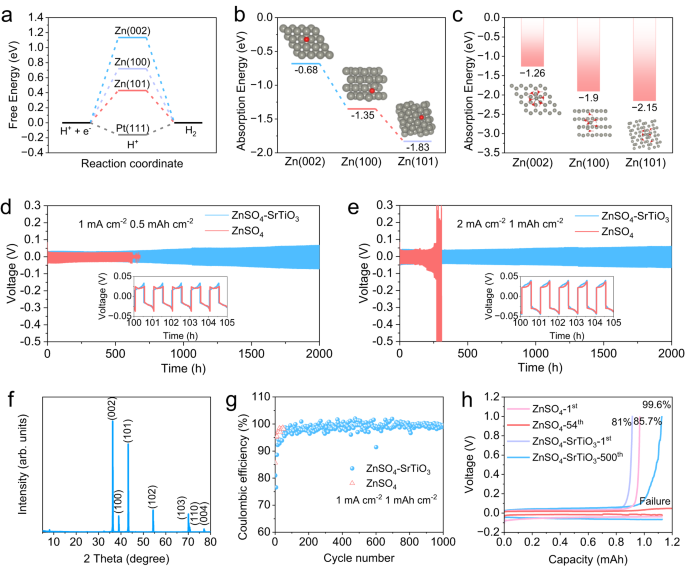
Additional, DFT outcomes show that the Zn (002) airplane not solely inhibits dendrite development but in addition inhibits HER and reduces floor corrosion. The calculated free power H adsorption displays the thermo-neutral adsorption, which may indicate a excessive exercise of HER40. As proven in Fig. 5a and Supplementary Fig. S10, the free power of Pt(111) is −0.16 eV, which may be very near thermo-neutral and HER can simply happen. The free energies of Zn (101), Zn (100), and Zn (002) are 0.43, 0.72, and 1.13 eV, respectively, suggesting that Zn (002) shouldn’t be conducive to H atom adsorption and thus successfully inhibits HER. Nevertheless, it’s value noting that Zn deposition is simpler alongside Zn (101) and (100) planes moderately than Zn (002), as a result of the adsorption power of Zn atoms at (002) is considerably larger than that of the opposite two crystal planes (Fig. 5b), indicating that the densified electrolyte can change the adsorption habits of zinc and expose extra crystal planes of (002). Along with inhibiting HER, Zn (002) additionally possessed glorious corrosion resistance. The calculation of the waste energies to strip the Zn atom from the zinc airplane exhibits that Zn (002) requires the very best power of 1.84 eV (Supplementary Fig. S11). The upper tripping-off power signifies a larger inner attraction between zinc atoms. Due to this fact, Zn (002) airplane possesses a robust chemical bond to suppress corrosion41. To additional examine the impact of corrosion inhibition, the adsorption energies of a typical solvation construction of Zn(H2O)62+ have been calculated. Zn (002) airplane has the very best energies of −1.26 eV, in comparison with that of the opposite two planes (Fig. 5c), which demonstrates that Zn (002) has nice potential to scale back by-products derived from solvated water molecules.
a The free-energy of HER on Zn (101), Zn (100), Zn (002), and Pt (111). b The absorption power of Zn atom at varied zinc crystal planes (the grey ball represents the Zn atom within the planes, the pink one is the free Zn atom). c The absorption power of Zn(H2O)62+ at varied zinc crystal planes (the grey ball represents the Zn atom within the planes, the opposite cluster is Zn(H2O)62+). Zn/Zn symmetric cells operated at completely different situations: d 1 mA cm−2, 0.5 mAh cm−2 and e 2 mA cm−2, 1 mAh cm−2. f The XRD patterns of the zinc foil after 50 h cycle. g The Coulombic effectivity of Zn/Ti half cells. h The corresponding charge-discharge curves at completely different cycles.
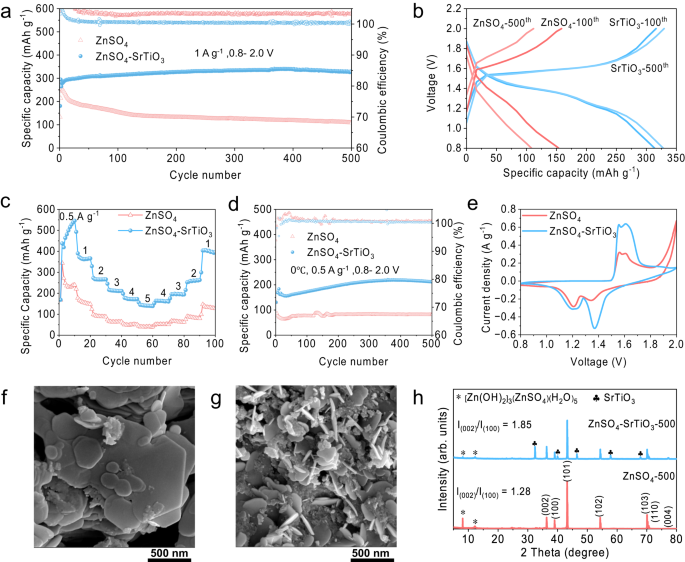
Because of inhibiting dendrite, HER, zinc corrosion, and by-products, the Zn/Zn symmetrical cells and Zn/Ti half cells exhibit glorious electrochemical efficiency in densified electrolytes. Zn/Zn symmetric configurations have been carried out to review the electrochemical stability of zinc metallic anodes in varied electrolytes. The cell utilizing typical electrolytes reveals a brief lifespan of 620 h below a small galvanostatic situation of 1 mA cm−2 and 0.5 mAh cm−2, in distinction to greater than 2000 h of the cell in densified electrolyte (Fig. 5d). Nevertheless, the overpotential of the cell utilizing the densified electrolyte is barely bigger than that of the cell utilizing typical electrolyte, which could be ascribed to the comparatively lowered ion conductivity of the densified electrolyte. When elevating the present density from 1 mA cm−2 to 2 mAh cm−2, the overpotential of the cell utilizing densified electrolyte is elevated appropriately, however the cycle stability remains to be assured. The symmetric cell can nonetheless be stably cycled for greater than 2000 h at 2 mA cm−2 within the densified electrolyte, whereas the overpotential will increase sharply after solely 200 h within the typical electrolyte (Fig. 5e). The drastically growing overpotential could be attributed to the deterioration of the interface because of massive accumulation of by-products produced by facet reactions within the typical electrolyte. In distinction, a slight improve in overpotential can be present in symmetric cells with densified electrolytes, that is attributable to settlement of SrTiO3 particles on the floor of zinc foils throughout a long-time cycle. The speed efficiency of the symmetric cell utilizing densified electrolyte was studied from 0.1 to 10 mA cm−2, which achieves a small overpotential of 79 mV at 5 mA cm−2 and 139 mV at 10 mA cm−2 (Supplementary Fig. S12). To research the deposition habits of zinc ions within the cell using densified electrolyte, a zinc anode was obtained from the cell cycled 50 h at 1 mA cm−2 for XRD and SEM checks. The XRD sample demonstrates that essentially the most uncovered crystal airplane is Zn (002), and no section of any by-product could be noticed, which is according to earlier outcomes (Fig. 5f). And the corresponding SEM photos show a easy layered stacked floor, in sharp distinction to that of zinc anode obtained from typical electrolyte, which reveals an uneven floor with dendrites and by-products (Supplementary Fig. S13). The Coulombic efficiencies (CEs) of Zn plating/stripping, one of the crucial essential parameters chargeable for the reversibility of electrochemical reactions, was studied by Zn/Ti half cells42. Within the densified electrolyte, the CEs progressively improve from the primary cycle, and a gentle cycle of effectivity above 99% is achieved after about 100 cycles, which lasts for greater than 1000 cycles (Fig. 5g). Nevertheless, though the cell utilizing typical electrolyte reveals the next CE within the first cycle, the cell failed after solely 54 cycles, more than likely because of quick circuits attributable to dendrite development. Quite the opposite, Fig. 5h exhibits that the Coulombic effectivity of the cell utilizing densified electrolyte is 81% within the first cycle, then progressively will increase to 99.6% within the five hundredth cycle, and the excessive CEs could be stably maintained for greater than 1000 cycles. As the rise of the cycle quantity, the overpotential will increase barely, which is due to the partial settlement of SrTiO3 on the electrodes because of the long-time operation. The long-term cycle stability of Zn/Zn symmetric cells at completely different present densities in addition to the excessive Coulomb effectivity of Zn/Ti half-cells point out that the densified electrolyte offers appreciable sensible software potential in aqueous rechargeable batteries.
Electrochemical efficiency of high-voltage Zn/MnO2 full cells
To show the sensible software of the densified electrolyte, Zn/MnO2 full cells have been assembled utilizing the standard electrolyte (2 M ZnSO4 and 0.1 M MnSO4) and the densified electrolyte. In response to the outcomes of the electrochemical stability window, the densified electrolyte has a big impact on inhibiting the electrolyte decomposition above 1.8 V. Due to this fact, the Zn/MnO2 full cells are charged to the next voltage of two.0 V. Because of this, the complete cell reveals a big improve in particular capability. To research the impact of SrTiO3 content material on the electrochemical efficiency, Zn/MnO2 full cells utilizing typical electrolytes and with varied SrTiO3 contents have been assembled. As proven in Supplementary Fig. S14, the cells utilizing typical electrolytes present a drastic decay, with a capability retention price of 43% after 500 cycles at a present density of 1 A g−1. In distinction, densified electrolytes can considerably enhance the precise capability and extra SrTiO3 particles are useful to capability retention. Nevertheless, extreme content material (60 wt%) results in extreme densification with seen reductions of mobility and conductivity, resulting in poor electrochemical efficiency. The cell with 50 wt% SrTiO3 reveals the perfect cycle stability in densified electrolytes, demonstrating an preliminary particular capability of 278.8 mA h−1 and a barely elevated particular capability of 328.2 mAh g−1 at five hundredth cycle (Fig. 6a). Their corresponding charge-discharge curves point out that there’s a marked lower of the voltage polarization within the densified electrolyte, suggesting a greater redox platform and sooner response kinetics, in comparison with the massive polarization in typical electrolyte (Fig. 6b). It’s value mentioning that the precise capability of Zn/MnO2 full cells with densified electrolyte has exceeded the theoretical worth of 308 mAh g−1, which is as a result of the Mn2+ from MnSO4 additive within the densified electrolyte is oxidized when the cell is charged to 2 V, thus contributing further capability past the unique stable MnO2. The redox response of Mn2+ within the densified electrolyte originates from the pH elevated from 3.4 to five.76 after the addition of SrTiO3 (Supplementary Fig. S15), leading to an extra electrochemical response as Eq. (2)43,44,45:
$${{{{{{rm{xZn}}}}}}}^{2+}+{{{{{{rm{yMn}}}}}}}^{2+}+{{{{{{rm{H}}}}}}}_{2}{{{{{rm{O}}}}}}, leftrightharpoons , {{{{{{rm{Zn}}}}}}}_{{{{{{rm{x}}}}}}}{{{{{{rm{Mn}}}}}}}_{{{{{{rm{y}}}}}}}{{{{{rm{O}}}}}}+2{{{{{{rm{H}}}}}}}^{+}+(2-2{{{{{rm{x}}}}}}-2{{{{{rm{y}}}}}}){{{{{{rm{e}}}}}}}^{-}$$
(2)
a Lengthy-term galvanostatic biking efficiency in varied electrolyte at a present density of 1 A g−1. b Corresponding cost and discharge curves at one centesimal and five hundredth cycle. c The speed efficiency of the complete cells. d Lengthy-cycling efficiency at 0 °C. e The cyclic voltammetry (CV) profiles at a scan price of 1 mV s−1. The SEM photos and XRD patterns of the anodes after 500 cycles in several electrolytes: f Densified electrolyte and g Standard electrolyte, and h The comparability of corresponding XRD patterns.
To exclude the interference of the energetic materials MnO2, the cathode containing solely Tremendous P and PVDF is designed to assemble the cell (be aware as SP cell). It’s discovered that SP cells can solely make further capability contributions when the densified electrolytes include MnSO4, ZnSO4, SrTiO3 and are charged to 2.0 V (Supplementary Figs. S16 and S17). The charge-discharge curves of the SP cell utilizing typical electrolytes have distinct plateaus round 1.99 V, which is attributable to the oxidation of small quantities of Mn2+ to MnO246. In distinction, the cells with densified electrolytes have an extended platform beneath 1.7 V, and the curve is slowly raised to 2.0 V. That is according to the charge-discharge curves in the entire Zn/MnO2 full cells (Supplementary Fig. S18), which signifies that the electrochemical habits of typical electrolyte is certainly completely different from the densified electrolyte. The cyclic voltammetry checks of the SP cells have been carried out to additional research the electrochemical habits. As proven in Supplementary Fig. S19a, after the primary cost to 2.0 V, the CV curves of SP cells with typical electrolyte are virtually the identical as that of Zn/MnO2 full cells, indicating that Mn2+ is oxidized to MnO2. Peculiarly, a weak peak similar to ZnxMnyO could be noticed on the third cycle of the CV curve, which is as a result of the pH will increase because of facet reactions, additional indicating that the ZnxMnyO can be produced in electrolytes with larger pH47,48. In distinction, the densified electrolyte demonstrates no sharp peaks at 2.0 V indicating virtually no MnO2 manufacturing, however distinct peaks at 1.65 and 1.35 V, which correspond to the redox response of ZnxMnyO (Supplementary Fig. S19b). The XRD patterns of cathodes of SP cells after charging to 2.0 V present additional proof (Supplementary Fig. S20). Within the typical electrolyte, the XRD sample solely exhibits the presence of the stainless-steel however with out MnO2, which can be because of the quantity is simply too small to be detected. In distinction, along with the height for chrome steel and SrTiO3 within the densified electrolyte, the XRD sample reveals a number of peaks, that are attributed to ZnxMnyO. Total, these outcomes present that the pH could be stabilized at about 5.8 after the addition of SrTiO3, which results in a steady and reversible response as Eq. (2), accompanied by further capability from the produced solids ZnxMnyO.
To show the sooner response kinetics, Zn/MnO2 full cells have been additional studied at varied present densities and a low temperature of 0 °C. Determine 6c compares the speed functionality of the cells in varied electrolytes. The densified electrolyte delivers a excessive particular capability of 463 mAh g−1 at 0.5 A g−1 and 144.9 mAh g−1 at the next present density of 5 A g−1. Nevertheless, solely 271.4 mAh g−1 at 0.5 A g−1 and 40.5 mAh g−1 at 5 A g−1 could be achieved within the typical electrolyte. Extra importantly, when the utilized present density returns to 1 A g−1, the precise capability of the cell utilizing densified electrolyte recovers to 392.6 mAh g−1. The upper particular capability in Fig. 6c than the capability in Fig. 6a is because of the presence of an electrochemical activation course of at a comparatively smaller present density, which is additional proved in Supplementary Fig. S22. In distinction, the precise capability solely recovers to 129.9 mAh g−1 within the typical electrolyte. Moreover, their corresponding charge-discharge curves show a smaller voltage polarization within the densified electrolyte (Supplementary Fig. S23). The Zn2+ storage kinetics is additional investigated by galvanostatic charging and discharging checks with a present density of 0.5 A g−1 at a temperature of 0 °C. Standard electrolytes ship a low preliminary particular capability of 86.5 mAh g−1 and 81.4 mAh g−1 on the five hundredth cycle, whereas the next preliminary particular capability of 130.8 mAh g−1 and an elevated worth of 212.6 mAh g−1 after 500 cycles are achieved within the densified electrolyte (Fig. 6d). The total growth and steady enchancment of the batteries capability at low temperature signifies the superb electrochemical response kinetics of the densified electrolyte, which could be attributed to the numerous enchancment of Zn2+ transference quantity. To be extra sensible, full cells with a excessive mass loading of 4 mg cm-2 have been studied in Supplementary Fig. S21. The cell utilizing densified electrolyte delivers a particular capability of 238 mAh g−1 after 200 cycles at 0.5 A g−1 with secure CEs. In distinction, the cell utilizing the standard electrolyte solely reveals a low particular capability and fails within the a hundred and seventieth cycle. To confirm the business availability of the densified electrolyte, pouch cells have been additionally assembled and studied at a continuing present density of 0.5 A g−1. The produced Zn/MnO2 pouch cells possesses the next open circuit voltage of 1.43 V than the coin cells and achieves an excellent particular capability of 367.2 mAh g−1 after 50 cycles (Supplementary Fig. S24).
The cyclic voltammetry (CV) profiles have been collected at a scan price of 1 mV s−1 to confirm the electrochemical behaviors. Zn/MnO2 full cells utilizing each the standard electrolyte and the densified electrolyte exhibit related shapes with two {couples} of redox peaks, however the cathodic peak at 1.35 V considerably will increase in densified electrolytes in comparison with typical electrolytes, whereas the cathodic peak at 1.2 V doesn’t change, which is because of the further redox response contributing to the capability (xZn2+ + yMn2+ + H2O ⇋ ZnxMnyO + 2H+ + (2 − 2x − 2y)e−). Moreover, the battery utilizing the densified electrolyte reveals comparatively larger response present and discount potential, indicating sooner response kinetics, decrease overpotential, and higher reversibility (Fig. 6e)49. Subsequently, electrochemical impedance spectroscopy (EIS) demonstrates that the complete cells utilizing densified electrolytes have higher response kinetics and electrochemical interface with smaller cost switch impedance, in comparison with the cells utilizing typical electrolytes (Supplementary Fig. S25). Zinc metallic anodes have been obtained from the complete cells after 500 cycles to review the electrochemical deposition habits within the strategy of repeated charging and discharging. The SEM picture of the anode from typical electrolyte exhibits zinc deposits alongside Zn (100) and (101) planes because of the course being virtually vertical and accompanied by quite a few scattered by-products (Fig. 6g). In distinction, there’s a flat and uniform deposition morphology with a definite hexagonal construction of Zn (002) planes on the floor of the anode from the densified electrolyte (Fig. 6f). Moreover, the corresponding cross-sectional photos point out that the zinc anode utilizing densified electrolytes demonstrates a smoother and denser floor, whereas the deposition morphology of the anode is free and undulating in a traditional electrolyte (Supplementary Fig. S26). Their XRD patterns show that zinc is extra simply deposited alongside Zn (002) airplane within the densified electrolyte with little by-product technology, as evidenced by the elevated ratio of IZn(002)/IZn(100) from 1.28 to 1.85, in addition to the practically vanishing by-products (ZSH) peaks (Fig. 6h). These outcomes reveal that the densified electrolyte offers the benefits of bettering response kinetics, regulating directional Zn deposition, and inhibiting facet reactions, thus considerably bettering the electrochemical efficiency of aqueous zinc-ion batteries.
[ad_2]