[ad_1]
Fabrication and characterizations of nano-metal diborides
We obtain the structural transformation of steel disulphide into steel diboride (MS2 + B → MB2 + Sx) beneath a KCl–CsCl molten salt situation (Fig. 1a and Supplementary Desk 1). The gaseous sulfur-containing merchandise could include S2, S4, S8, and B2S3 on the response temperatures (1000–1100 °C), whereas S2 is taken into account to be essentially the most plentiful species31. We calculate the response enthalpy and entropic time period to find out the Gibbs free enthalpy of the chemical response (ΔG = ΔH − TΔS). As exhibited in Fig. 1b and Supplementary Desk 2, the entropic time period TΔS of the gaseous sulfur-containing merchandise is the dominant a part of the entire Gibbs power. After reaching a sure temperature, the entropy achieve would drive the response from a thermodynamically unfeasible course of to a thermodynamically spontaneous course of. In accordance with the entropy-driven route, we are able to synthesize 9 monometallic diborides, together with MB2 (M = Ti, Zr, Hf, V, Nb, Ta, Mo, W, and Re). The powder X-ray diffraction (XRD) patterns in Fig. 1c affirm that almost all of as-synthesized samples are pure transition steel diboride phases. The morphologies of those borides are revealed by scanning electron microscopy (SEM, Supplementary Fig. 2). TaB2 and MoB2 possess a typical nanosheet construction, WB2 exhibits a nanowire morphology, and different MB2 samples (M = Ti, Zr, Hf, V, Nb, and Re) are composed of nanoparticles with sizes starting from 50 to 200 nm. Most of those MB2 samples possess giant Brunauer–Emmett–Teller (BET) floor areas inside the vary of 40–60 m2 g−1 (Fig. 1d and Supplementary Desk 3), that are clearly increased than these of borides obtained by typical high-temperature ceramic technique32,33,34,35,36.
a A schematic presenting the synthesis and crystal construction of TaB2. b Temperature-dependent Gibbs free power of the synthesis means of steel diborides. c XRD patterns of as-synthesized diborides samples. For comparability, the Joint Committee on Powder Diffraction Customary (JCPDS) playing cards of those steel diborides are included. d N2 adsorption-desorption isotherms of diborides samples. e TEM picture and (f) HRTEM picture of TaB2. The inset of Fig. 1f exhibits SAED sample of TaB2.
We’ve in contrast the acid corrosion resistance and BET floor space of 9 steel diborides synthesized above. In contrast with TaB2, the TiB2, ZrB2, HfB2, VB2, CrB2, MoB2, and WB2 present extra extreme steel dissolution in acid (Supplementary Desk 3). After the publicity to 0.5 M H2SO4 over per week (Supplementary Fig. 3), nearly no Ta species are leached within the acidic resolution, and TaB2 retains its pristine crystal construction properly. These outcomes suggest the excellent chemical stability of TaB2 in acid. As well as, TaB2 presents a lot increased BET floor space than HfB2, VB2, NbB2, and ReB2 (Supplementary Fig. 3). From the crystal construction perspective, the TaB2 includes 3D metallic Ta–Ta framework and 2D graphene-like boron layers (often known as borophene subunits), suggesting a quick electron transport property37. Experiments current TaB2 has a excessive conductivity of 25.2 S cm−1 (Supplementary Fig. 4), which is considerably bigger than these of previously-reported helps of IrO238,39,40,41, comparable to TiN (3.9 S cm−1), TaC (0.65 S cm−1) and TiO2 (1.8 × 10−4 S cm−1). Given its multi-advantages together with giant BET floor areas, nice acid corrosion resistance, and excessive conductivity, the nano-TaB2 is specifically chosen to discover the potential as assist of IrO2 catalysts. Furthermore, the crust abundance of Ta is 5 orders of magnitude increased than that of Ir (Supplementary Desk 4), and Ta prices round 2‰ of the Ir value ($367.5 for Ta vs. $164,662.0 for Ir per kilogram in 2023). Therefore, the introduction of TaB2 supporting materials is predicted to scale back the price of the anode catalyst layer and enhance the feasibility of PEMWE.
We additional characterised the TaB2 pattern with SEM and transmission electron microscopy (TEM). The SEM (Supplementary Fig. 2f) and TEM pictures (Fig. 1e) current hat TaB2 nanosheets possess an edge size of 200–500 nm and a thickness of 5–20 nm. Excessive-resolution TEM picture (HRTEM) of TaB2 nanosheet (Fig. 1f) reveals its good crystallinity. Within the HRTEM picture and the corresponding selected-area electron diffraction (SAED) sample (Fig. 1f, inset), interplanar spacing of 0.268 nm might be noticed for 3 units of lattice fringes, assigning to {001} planes of TaB2 part. The angle of 60o between the three planes is per the theoretical worth. Furthermore, N2 adsorption-desorption isotherms of TaB2 show typical type-II curves with H3 hysteresis loop (Fig. 1d), suggesting the presence of interlaminar pores amongst adjoining nanosheets. The BET floor space of TaB2 is calculated to be 54.6 m2 g−1. These outcomes reveal the profitable synthesis of high-quality TaB2 nanosheet with giant floor areas.
Loading IrO2 nanocatalysts on boride helps
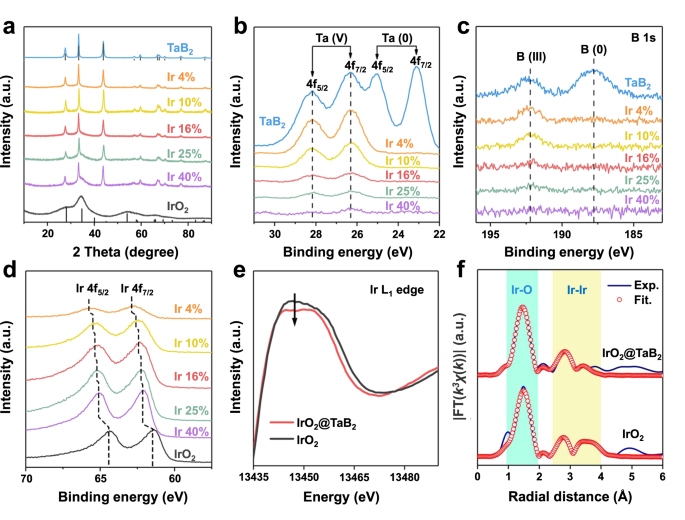
The loading of IrO2 nanocatalysts on TaB2 helps (IrO2@TaB2) is achieved via calcinating a mix of Ok2IrCl6 and TaB2 in molten NaNO3 (i.e., 350 °C). The loading quantities of IrO2 will be managed by altering the feeding of the iridium supply. When TaB2 just isn’t employed within the synthesis, pure IrO2 nanoparticles with a dimension of 1.4–2.0 nm are shaped (Supplementary Fig. 5 and 6). A sequence of IrO2@TaB2 samples with Ir contents of 4 wt%, 10 wt%, 16 wt%, 25 wt%, and 40 wt% are synthesized, and their iridium contents are quantified by inductively coupled plasma atomic emission spectroscopy (ICP-OES). The XRD patterns of those IrO2@TaB2 samples are proven in Fig. 2a, with these of TaB2 and unsupported IrO2 as references. The diffraction peaks of TaB2 helps dominate the XRD patterns of IrO2@TaB2. As well as, a broadened peak at 30-40o will be attributed to IrO2, suggesting the very small dimension of IrO2. With the rise in IrO2 contents on TaB2 helps, the diffraction intensities of IrO2 enhance progressively.
These IrO2@TaB2 samples are additional characterised by X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS). Determine 2b exhibits Ta4f XPS spectra of IrO2@TaB2 samples and the reference TaB2. For TaB2, the floor includes each metallic Ta parts and oxidized Ta parts, indicating slight floor oxidation. Not like that of TaB2, the floor layers of IrO2@TaB2 samples are absolutely dominated by oxidized Ta parts, suggesting additional floor oxidation of Ta species through the IrO2 loading course of. With growing IrO2 content material within the samples, the sign intensities of Ta4f XPS lower progressively, implying the lower of floor Ta focus. Equally, the floor B focus additionally decreases with growing IrO2 content material and is nearly undetectable after the 16% Ir content material (Fig. 2c). Based mostly on the Ta, B and Ir XPS sign intensities (Fig. 2b–d), we conclude that the Ta and B species dominate the surfaces of IrO2@TaB2 samples with the Ir loading content material ≤10%; when the Ir loading content material >10%, the Ta and B sign intensities are very weak and the Ir species dominate the surfaces, indicating a dense coating of IrO2 shaped on the assist floor. As well as, the Ir4f XPS peaks of IrO2@TaB2 samples (Fig. 2nd) current an apparent shift to bigger binding power relative to these of IrO2, and the deviation will increase with the lower in IrO2 contents on TaB2 helps. This means a barely decrease Ir oxidation in IrO2@TaB2 as a result of digital interplay between IrO2 and assist. The digital interplay and Ir oxidation state will be flexibly regulated by the Ir loading content material.
The cost redistribution of IrO2 can be supported by X-ray absorption near-edge construction (XANES) spectra. We word that we selected 16 wt% IrO2@TaB2 as a consultant pattern, which displays an optimized iridium mass exercise towards OER in an ordinary three-electrode cell. Except in any other case specified, subsequent IrO2@TaB2 refers back to the pattern with 16 wt% Ir loading. We additionally word that we selected Ir L1-edge for the XANES measurements, contemplating that the Ir L3-edge overlaps with the Ta L2 and Ta L1-edges. Determine 2e exhibits the Ir L1-edge XANES spectra of IrO2@TaB2 and IrO2. In comparison with that of IrO2, the white-line power place of IrO2@TaB2 has a barely weaker peak depth, indicating an elevated occupation in 5d electron orbital and a comparatively decrease Ir oxidation state in IrO2@TaB2. Furthermore, prolonged X-ray absorption fantastic construction (EXAFS) displays comparable structural info of IrO2@TaB2 and IrO2, and the corresponding becoming outcomes (Fig. 2f and Supplementary Desk 5) present that each the Ir-Ir and Ir-O bond distances in IrO2@TaB2 are near these in IrO2. In accordance with the above XPS and XAS outcomes, we are able to fairly conclude that: (i) the lattice of IrO2 has nearly unchanged after loading on TaB2; (ii) nevertheless, the interfacial digital interplay between IrO2 and assist results in the cost redistribution of IrO2.
The aberration-corrected high-angle annular dark-field scanning TEM (HAADF-STEM) was utilized to analyze IrO2@TaB2 pattern. As proven in Fig. 3a, the IrO2 nanoparticles are uniformly and tightly dispersed on the TaB2 sheets. The primary sizes of IrO2 are about 1.5 nm (Supplementary Fig. 6). The power dispersive X-ray spectrum (EDX) elemental mappings of IrO2@TaB2 pattern in Fig. 3b and Supplementary Fig. 7 affirm that Ta and B parts present sheet morphology, whereas Ir and O parts are homogeneously distributed in helps. The facet view of TEM picture (Fig. 3c) reveals that an round 5 nm floor coating of IrO2 nanocrystallites is shaped on TaB2 helps. The amorphous filler amongst IrO2 nanocrystallites will be attributed to TaOx layer on TaB2 surfaces. Each the facet view and high view of TEM pictures (Fig. 3c, d) present that the IrO2 nanocrystallites are randomly however spatially interconnected. The HRTEM picture (Fig. 3e) presents lattice fringes of the (11(bar{1})) aircraft (~0.224 nm) and (020) aircraft (~0.225 nm) of IrO2, respectively. The angle between the 2 planes is set as 59.1o, equal to the theoretical worth.
Based mostly on the above electron microscopy, electron diffraction, and spectroscopic proof, the structural illustration of IrO2@TaB2 pattern is proven in Fig. 3f. First, the floor of TaB2 assist tends to type amorphous TaOx layer. Second, the IrO2 nanocrystallites are uniformly and tightly dispersed on the helps to type 5 nm thick IrO2 layers. Third, the IrO2 nanocrystallites with intrinsic conductive properties are spatially interconnected, and the IrO2 is electrically related to the metallic TaB2 assist, making certain the formation of conductive networks. Fourth, the interfacial digital coupling in TaOx/IrO2 catalytic layer leads to the valence state discount of Ir atoms.
Electrocatalytic efficiency in a three-electrode configuration
The electrocatalytic actions towards OER of IrO2@TaB2 had been measured utilizing an ordinary three-electrode configuration in 0.1 M HClO4 electrolyte. Because the IrO2 content material will increase, the electrocatalytic exercise for OER elevated quickly (Fig. 4a), and reaches its optimization when the Ir content material is 16 wt%. Nevertheless, an additional enhance within the Ir content material of the IrO2@TaB2 leads to a slight deterioration of the electrocatalytic exercise. The required overpotential to generate a present density of 10 mA cm−2 (normalized over the electrode geometric space) for 16 wt% IrO2@TaB2 is 288 mV, decrease than that of unsupported IrO2 (307 mV). The Tafel slope of 16 wt% IrO2@TaB2 is 42.6 mV dec−1 (Supplementary Fig. 8), near that of unsupported IrO2 (45.1 mV dec−1), indicating that the the TaB2 assist has no change to OER pathway of IrO2.
a The polarization curves towards OER within the presence of IrO2@TaB2 and IrO2 in 0.1 M HClO4. b Comparability of ECSA and present densities (jECSA) normalized by ECSAs for IrO2@TaB2 and IrO2. c Comparability of iridium mass actions (jIr) of Ir-based electrocatalysts at 1.53 V versus RHE40,42,43,44,45,46,47,48,49,50,51,52. d Chronopotentiometric curves of IrO2@TaB2 and IrO2 with a present density of 10 mA cm−2. e Contents of leached iridium within the electrolyte within the presence of IrO2@TaB2 and IrO2 throughout electrocatalysis. f The polarization curves for OER of IrO2@TaB2 and IrO2 in HClO4 electrolyte with completely different pH. g pH dependence of IrO2@TaB2 and IrO2 on the OER potential on the SHE scale. h DEMS alerts of O2 merchandise for 18O-labeled IrO2@TaB2 in 0.1 M HClO4 in H216O.
Electrochemical double layer capacitance (DLC) measurements had been carried out to acquire the electrochemically lively floor space (ECSA) of IrO2@TaB2 by estimating the gathered cost quantity on the electrode floor. Typically, the ECSA values turn into bigger with growing Ir content material in IrO2@TaB2 (Fig. 4b, left and Supplementary Desk 6). With a purpose to examine the intrinsic actions of various catalysts, we normalized the measured currents by the ECSAs (Fig. 4b, proper). The 16 wt% IrO2@TaB2 presents the very best present density (jECSA) at 1.53 V versus reversible hydrogen electrode (RHE) amongst these IrO2@TaB2 samples, which is 5.2 occasions bigger than that of IrO2. We additional in contrast the iridium mass actions of IrO2@TaB2 and IrO2 by normalizing the measured currents over the mass of iridium (jir). Though 16% IrO2@TaB2 has 70 wt% much less iridium relative to IrO2, it shows a excessive iridium mass exercise of 345 A g−1 at 1.53 V versus RHE (Fig. 4c and Supplementary Desk 7), which is an order of magnitude increased than that of IrO2. The iridium mass exercise of 16 wt% IrO2@TaB2 is superior to some recently-reported iridate electrocatalysts (e.g., 3C-SrIrO3, 6H-SrIrO3, Sr2IrO4, Ok0.25IrO2)42,43,44,45,46,47 and people consultant supported catalysts (e.g., IrO2-TiO2, IrO2@Ir/TiN, IrO2/Nb0.2Ti0.8O2)40,48,49,50,51,52.
Apart from excessive exercise, IrO2@TaB2 shows nice catalytic and structural stability for acidic OER. The galvanostatic measurements (Fig. 4d) reveal that IrO2@TaB2 stays steadily catalytic exercise for greater than 120 h of steady operation at a present density of 10 mA cm−2, whereas IrO2 misplaced its catalytic exercise after 40 h within the acidic electrolyte. Supplementary Fig. 9 displays the polarization curve for OER obtained from IrO2@TaB2 and IrO2 earlier than and after 5000 cycles. IrO2@TaB2 presents no measurable lack of catalytic exercise after the continual polarization measurements, whereas IrO2 suffers from a extreme deactivation. These outcomes counsel that TaB2 assist tremendously enhances the catalytic stability of IrO2. Furthermore, IrO2@TaB2 achieves a faradaic effectivity of almost 100% throughout acidic OER (Supplementary Fig. 10), confirming that the noticed present will be totally attributed to the oxygen technology.
The leached quantities of cations throughout OER of IrO2@TaB2 and IrO2 had been quantitatively decided by ICP-OES (Fig. 4e). There are not any detectable leached Ta and B species for IrO2@TaB2 within the electrolyte, and the Ir dissolutions enhance through the first two hours after which reaches a steady state. The fixed Ir focus for IrO2@TaB2 within the electrolyte is 0.25 mg L−1, which is far weaker than that for IrO2 (0.4–0.7 mg L−1), suggesting that the dispersion of IrO2 on TaB2 helps markedly improves the structural stability. The steadiness of IrO2@TaB2 is additional evaluated by calculating the soundness quantity (i.e., S-number proposed by Gieger et al.)53, which is an efficient metric that relates the quantity of developed oxygen to the dissolved iridium. As proven in Fig. 4e (inset), the S-number of IrO2@TaB2 through the acidic OER is 5.2 × 104, which is increased than that of IrO2 (2.9 × 104). As well as, IrO2@TaB2 nonetheless maintains the preliminary morphology and construction after OER, as supported by HRTEM, XRD, and XPS outcomes (Supplementary Fig. 11–13). These outcomes total affirm the wonderful electrocatalytic and structural stability of IrO2@TaB2 towards acidic OER.
Catalytic mechanism and origin of excessive exercise
To analyze the catalytic mechanism, OER efficiency of IrO2@TaB2 and IrO2 had been examined in HClO4 electrolytes with completely different pH. The catalytic actions of IrO2@TaB2 and IrO2 present sturdy pH dependence within the pH vary of 0–1.5 on the usual hydrogen electrode (SHE) scale (Fig. 5a). The potential–pH dependence worth of IrO2@TaB2 is −57.3 mV dec−1, which is close to to that of IrO2 (−58.0 mV dec−1, Fig. 5b). Such a close to −60 mV dec−1 Nernstian potential shift signifies a proton coupled electron switch (CPET) course of54, and the OER at Ir websites strictly follows the standard adsorbates evolution mechanism (AEM) pathway. As well as, we utilized in situ differential electrochemical mass spectrometry (DEMS) to review the response mechanisms. The O supply of developed oxygen product will be recognized via labeling of catalysts with 18O isotope. When the 18O-labeled IrO2@TaB2 electrocatalyst works in H216O electrolyte, 34O2 (or 16O18O) to 32O2 ratio in gaseous product is 0.43% (Fig. 5c). It needs to be famous that the pure steady abundance of 18O isotope is about 0.2% in water55,56, indicating the minimal detected quantity of 34O2 in OER merchandise is about 0.4%. We additional carried out DEMS measurement for 18O-labeled IrO2 catalyst beneath the identical situation, the 34O2/32O2 depth ratio from the response merchandise can be 0.43% (Supplementary Fig. 14). These outcomes total reveal that like that of well-known IrO2 catalysts, the OER mechanism of IrO2@TaB2 catalyst strictly undergoes the adsorbate evolution mechanism (AEM), excluding the participation of lattice oxygen through the electrocatalysis.
a Stack construction and key supplies of a PEMWE. b Cross-section SEM picture of CCM using 40% IrO2@TaB2 anode layer and 40% Pt/C cathode layer. c Polarization curves of PEMWEs utilizing IrO2@TaB2 and IrO2 anodes at 80 °C with Nafion 115 membrane. d Comparability of present densities of PEM electrolyzers utilizing completely different iridium-based catalysts at a cell potential of 1.9 V16,18,60,61,62,63,64,65,66,67,68,69. e Chronopotentiometry curve of PEMWEs utilizing IrO2 and IrO2@TaB2 anodes operated at 1 A cm−2. f, g EIS curves of PEMWEs utilizing IrO2@TaB2 and IrO2 anodes. h The comparability of ohmic resistance, activation resistance, and diffusion resistance for PEMWEs utilizing IrO2@TaB2 and IrO2 anodes. The inset exhibits EEC mannequin for EIS becoming.
After figuring out the catalytic mechanism, we sought to elucidate why IrO2@TaB2 possesses excessive exercise for OER. On condition that the crystal lattice and catalytic mechanism of IrO2 have nearly no change after loading on TaB2, the improved efficiency of IrO2@TaB2 for OER is interpreted as follows. (i) The common particle sizes of IrO2@TaB2 and IrO2 are estimated to be 1.5 nm and 1.8 nm (Supplementary Fig. 6). Confinement of IrO2 nanoparticles on the TaB2 helps reduces IrO2 dimension, which supplies extra lively websites to enhance OER exercise. (ii) The interfacial digital coupling in TaOx/IrO2 catalytic layer is chargeable for the excessive intrinsic exercise of IrO2@TaB2. A steel−semiconductor heterojunction is constructed between TaOx and IrO2 on TaB2 floor, ensuing within the formation of a floor electrical area and powerful digital interplay (Supplementary Fig. 15a). The electrons will stream from the conduction band of TaOx to IrO2, pushed by the work operate variations, resulting in electron-rich IrO2. The antibonding states of IrO2 are extra absolutely occupied by electrons within the IrO2-TaOx heterojunction, which lowers the floor oxygen adsorption of IrO2 and consequently boosts the OER exercise (Supplementary Fig. 15b, c). We word that the chemical and structural complexity of IrO2@TaB2 catalysts, together with the unsure floor constructions of IrO2 nanoparticles (with out particular uncovered aspects), amorphous TaOx layer, and their heterointerface, makes it unrealistic to determine a transparent structural mannequin of the catalyst. This limits us from precisely modeling the electrochemical processes of OER and quantitatively describing catalytic exercise by superior calculation strategies (e.g., grand-canonical DFT)57.
Efficiency of PEMWE gadgets
The efficiency of as-prepared IrO2@TaB2 as an anode catalyst was lastly evaluated on an actual PEM electrolyzer (Fig. 5a). Not like the three-electrode configuration to primarily mirror the efficiency of the catalyst itself, the PEMWE requires wonderful electrical conductivity of catalyst (larger than 0.1 S cm−1) to ship excessive present densities of a number of A cm−2 3,4. {The electrical} conductivity of IrO2@TaB2 turns into increased because the IrO2 content material will increase (Supplementary Fig. 16), and as much as 0.17 S cm−1 for the 40% IrO2@TaB2 pattern, which has similarities to the conductivity of IrO2 itself (0.18 S cm−1). The construction and composition characterizations of the 40percentIrO2@TaB2 pattern by ICP-OES, XRD, XPS (Fig. 2a–c), and TEM (Supplementary Fig. 17) affirm that there isn’t any important distinction between 40percentIrO2@TaB2 and 16percentIrO2@TaB2 pattern, apart from increased Ir loading of the previous. Therefore, the 40% IrO2@TaB2 pattern is employed because the optimum anode catalyst to combine into PEMWE. The Nafion 115 membrane is used because the PEM, which is a perfluorosulfonic polymer with a thickness of 125 μm. The CCM with a 5 cm2 working space (Supplementary Fig. 18) using 40% IrO2@TaB2 anode layer and 40% Pt/C cathode layer is produced by a decal switch technique. SEM pictures in Fig. 5b and Supplementary Fig. 19 exhibit the cross-section and high view morphology of the CCM. Each the catalyst particles of IrO2@TaB2 anode and Pt/C cathode are composed of uniformly distributed agglomerates on the membrane floor. The thickness of each anode and cathode catalyst layers on the membrane is roughly 5 μm. As analyzed by ICP-OES, the CCM incorporates a low Ir loading of 0.15 mg cm−2 on the anode layer and a low Pt loading of 0.27 mg cm−2 on the cathode layer (Supplementary Fig. 20), respectively. The CCM exhibits decrease whole loadings of PGMs (0.42 mg cm−2) than the DOE 2023 goal (1.0 mg cm−2) and even the DOE 2025 goal (0.5 mg cm−2)58.
Even such low noble metals utilized in our CCM, the polarization curve of PEMWE reveals a present density of three.06 A cm−2 with a cell potential of two.0 V, working at 80 °C and ambient strain (Fig. 5c). It’s stunning that the efficiency of our PEMWE has reached that of US DOE 2023 goal (1.9 V@2.5 A cm−2), beneath such low PGM loadings (0.15 mgIr cm−2 and 0.27 mgPt cm−2). We word that the normal CCMs in industrial PEMWE obtain cheap exercise and stability utilizing a excessive Ir loading of two–4 mgIr/cm2 to make sure enough in-plane conductivity and mechanical stability of catalyst layer59. In reality, the efficiency of PEMWE utilizing IrO2@TaB2 as anode electrocatalyst exceeds the newest studies of PEMWEs utilizing novel anode electrocatalysts (e.g., Sr2CaIrO6, Ta0.1Tm0.1Ir0.8O2-δ, Ir@WOx)16,18,60,61,62,63,64,65,66,67, as proven in Fig. 5d and Supplementary Desk 8. Even when a number of supported catalysts (e.g., Ir@B4C, Ir@Nb2O5-x) have reported to achieve the DOE 2023 goal, they require a number of occasions increased iridium loadings68,69.
We additional examine the efficiency of PEMWEs utilizing IrO2@TaB2 and industrial IrO2 as anode electrocatalysts (Fig. 5c), beneath the same Ir loading in CCM. Whereas IrO2 using as anode layer, the PEMWE delivers a considerably decrease present density of 1.9 A cm−2 relative to IrO2@TaB2 catalyst (3.06 A cm−2) at 2. 0 V cell potential. To judge catalyst stability, the IrO2 and IrO2@TaB2 anode cells had been examined at a continuing present density of 1 A cm−2. The PEMWE utilizing IrO2 anode undergoes a extreme deactivation beneath the low Ir loading of 0.2 mg cm−2, and the IrO2 anode cell displays cheap stability when the Ir loading will increase to 2 mg cm−2 (Fig. 5e and Supplementary Fig. 21–24). By comparability, the PEMWE utilizing IrO2@TaB2 anode supplies a gradual operation for greater than 120 h beneath a low Ir loading of 0.15 mg cm−2 (Fig. 5e), confirming wonderful catalytic stability. We are able to speculate that the IrO2@TaB2 with bigger materials quantity can varieties a thicker catalyst layer relative to reveal IrO2, in order that the previous possesses enough layer conductivity and stability at such a low Ir loading. The good exercise and stability reveal the good potential of IrO2@TaB2 as a sensible anode of actual PEMWE for industrial software.
To infer the origin of efficiency variations, we feature out chemical impedance spectroscopy (EIS) experiments on PEMWEs utilizing IrO2@TaB2 and IrO2 as anode electrocatalysts, respectively (Fig. 5f, g). We additional match the EIS Nyquist plots by equal electrical circuit (EEC) mannequin (Fig. 5h, inset), which exhibits good accordance with the experimental outcomes. The entire losses in a PEMWE primarily compose of ohmic resistance, activation resistance, and diffusion resistance70,71. (i) The Rcell denotes the cell ohmic resistance, which is expounded to ohmic losses of all elements together with membrane, catalyst layers, porous transport layers (PTL), bipolar plate, and their interfacial resistances. The RCell is nearly unaffected at completely different present densities for IrO2@TaB2 anode cell, which will be mirrored by excessive frequency resistance (HFR, Supplementary Fig. 25). The IrO2@TaB2 anode cell exhibits a slight lower in ohmic resistance relative to the IrO2 anode cell. (ii) The Rac denotes the activation resistance to find out the response kinetics of anode and cathode electrocatalysts. Rac is delicate to present density and is especially contributed from the anode. The Rac of IrO2@TaB2 anode cell is considerably decreased in contrast with that of IrO2 anode cell, as a result of significantly better catalytic efficiency IrO2@TaB2 relative to IrO2. (iii) The Rd denotes the diffusion resistance, as mirrored by the Warburg diffusion aspect (Wd). Rd is negligible at a low present density and will increase with the rise of present density. The IrO2@TaB2 anode cell presents a lot decrease transport loss in contrast with the IrO2 anode cell. Taken total, it may be concluded that the IrO2@TaB2 anode cell displays simultaneous decreases in each ohmic, activation, and diffusion losses relative to the IrO2 anode cell.
[ad_2]