
[ad_1]
Growing applied sciences that may convert CO2 into artificial fuels and base chemical compounds is of key significance for reaching local weather objectives. The electrochemical discount of CO2 at copper electrodes utilizing electrical energy from renewable sources might be used for the manufacturing of e-fuels. New research present that this course of adjustments the association of the copper atoms on the catalyst floor.
Copper is an indispensable catalyst materials for CO2 discount, particularly for the synthesis of specific beneficial chemical compounds and fuels, resembling ethanol. It’s extremely favorable if the copper atoms on the floor have a strongly disordered association, and might be achieved, for instance, by pre-oxidizing the copper floor or via alloying.
A joint examine of the Institute of Experimental and Utilized Physics of Kiel College and the Division of Interface Science on the Fritz Haber Institute (FHI) of the Max Planck Society (MPG) confirmed that such disordered constructions additionally fashioned spontaneously within the very preliminary levels of the electrocatalytic CO2 discount response.
The researchers noticed that copper atoms contained in the steel moved towards the floor and assembled right into a freestanding group of just a few atoms. This transformation of the steel was attributable to CO, an intermediate product of the response, and was preserved even at excessive response charges. The outcomes have been printed right now within the journal Nature Catalysis.
“As a result of the electrocatalytic CO2 discount happens at metals in aqueous carbonate resolution much like mineral water, and is accompanied by the formation of hydrogen gasoline, detailed investigations of the method on the atomic scale is tough,” says Olaf Magnussen, Professor for Stable State Physics at Kiel College.
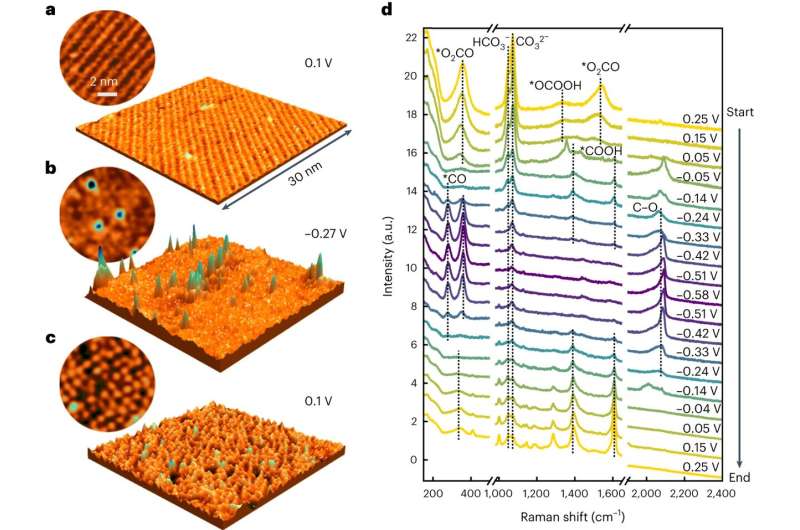
The researchers subsequently used a mixture of strategies that may be employed even beneath these difficult situations. The workforce from Kiel noticed the transformation of the copper floor immediately by high-resolution electrochemical scanning tunneling microscopy. This methodology permits direct imaging of the atoms and molecules on the floor.
X-ray diffraction research, carried out by scientists from Kiel and Berlin on the PETRA III synchrotron of DESY in Hamburg confirmed this transformation. As well as, these measurements confirmed that at excessive response charges the freestanding copper atoms have been maintained, however no additional formation occurred. Molecular spectroscopy on the Fritz Haber Institute lastly indicated that the adjustments have been induced by the fashioned CO.
“The outcomes recommend a drastic transformation of the electrode floor every time that the electrical present required for the CO2 discount is switched on. This was beforehand unknown, however might play an essential function in catalysis,” says Professor Beatriz Roldán Cuenya, Director of the Interface Science Division at FHI.
One technique to affect the construction of the catalyst and thus the kind of fashioned chemical compounds is to function the electrode with voltage pulses. Certainly, this had been proven already in earlier work by the analysis workforce. Nonetheless, the copper there was periodically oxidized by the use of electrical power, which requires the polarity of the electrode to be reversed.
In line with the brand new outcomes, related results is likely to be obtained already by merely switching the present on and off. “General, the examine confirms our speculation that not solely the electrode materials but in addition the working situations and micro-environment are related for the belief of this environmentally pleasant know-how,” says the workforce.
Extra data:
Reihaneh Amirbeigiarab et al, Atomic-scale floor restructuring of copper electrodes beneath CO2 electroreduction situations, Nature Catalysis (2023). DOI: 10.1038/s41929-023-01009-z
Offered by
Christian-Albrechts-Universität zu Kiel
Quotation:
Observing microscopic transformations of electrocatalyst surfaces (2023, August 18)
retrieved 20 August 2023
from https://phys.org/information/2023-08-microscopic-electrocatalyst-surfaces.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.
[ad_2]