
[ad_1]
One of many essential features of chemical response mechanisms is the order during which issues occur. Extra particularly, the order during which bonds make or break when there are greater than two concerned in endeavor a response. So we now have:
- concerted mechanisms, when all bonds in any explicit stage of a mechanism are altering in live performance by way of a singular transition state,
- asynchronous concerted mechanism, when all of the bonds are altering, however not essentially all on the similar price and which can contain so referred to as “hidden intermediates”, however which nonetheless stil entails just one transition state.
- stepwise mechanisms, during which a couple of transition state is concerned, related by a discrete intermediate alongside the pathway.
Right here I contemplate an instance of one other kind of (isomerisation) mechanism, involving bond rotations moderately than bond formations or breakages. The 2 bonds on this case have a better bond order than 1, and so are beginning to verge on a sort of isomerism referred to as atropisomerism, the place the rotation takes place on a comparatively gradual time scale (not like single bonds themselves, the place rotation about them is often comparatively quick). Do two such bonds rotate in a stepwise or a concerted method? Within the construction beneath, we now have two rotatable bonds, proven in purple and blue, which on account of conjugation of the lone electron pair on the nitrogen atoms with the carbonyl group have bond orders >1. Do these bonds rotate in live performance or in a stepwise method?
The calculations of the rotations are executed on the B3LYP+GD3+BJ/Def2-SVPP/SCRF=DCM stage, Knowledge DOI: 10.14469/hpc/12299
- Firstly, for the system R=R’ = Me. The response coordinate is specified across the purple bond.
The animation alongside the IRC (Intrinsic response coordinate) seems beneath, the place you’ll be able to see the purple bond rotating and the blue bond spectating.
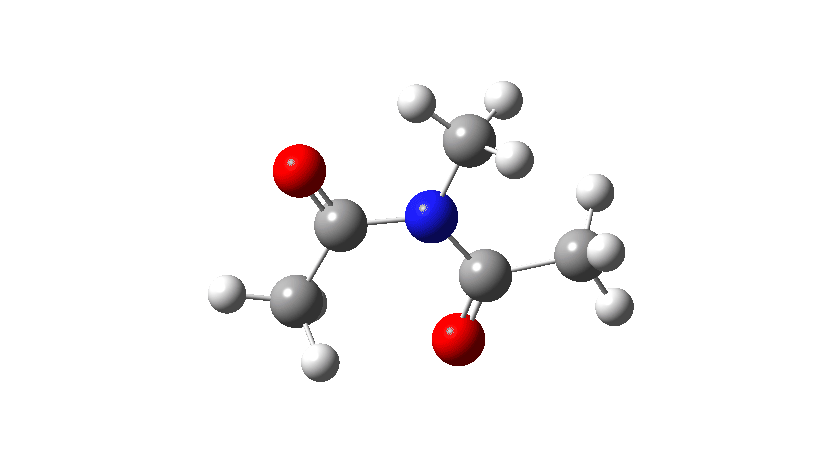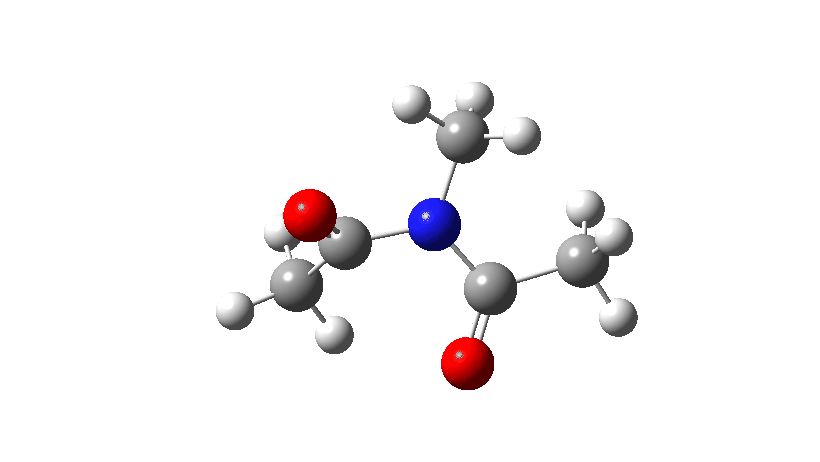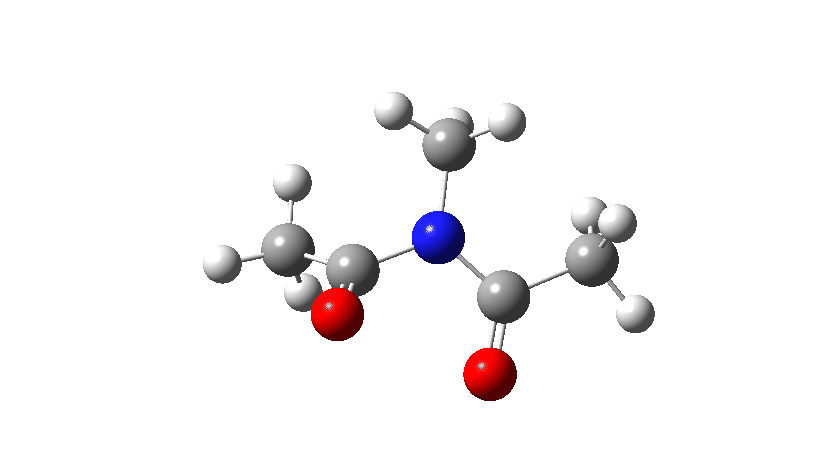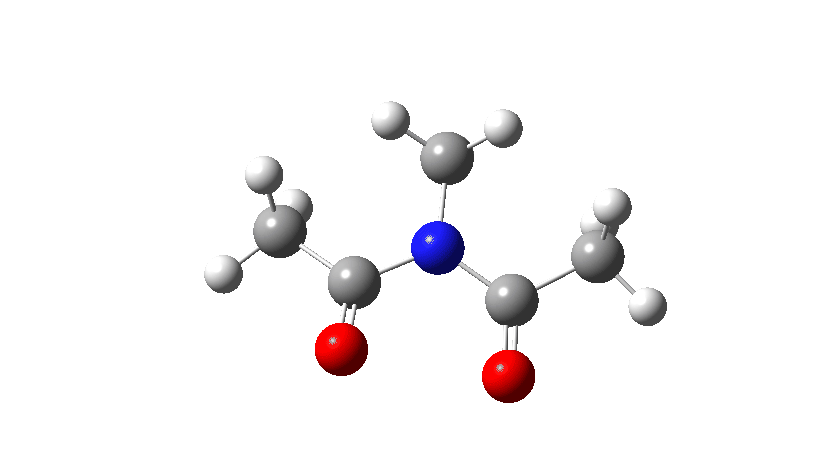
- The response of the dihedral angles about each bonds is proven beneath, which reinforces the conclusion that while one dihedral modifications by about 180°, the opposite hardly modifications. The general dipole second modifications considerably because of the relative orientation of the 2 carbonyl teams altering. The 2 bonds will be stated to rotate in a stepwise mechanism, involving an intermediate the place one has rotated and the opposite has not.
- When the majority of the central group is elevated, completely different behaviour is now noticed.
- Each dihedral angles now change by ~180°, in live performance however not in synchrony! The primary roughly transforms evenly by ~180°, however the second modifications path at ~IRC=-5 to rejoin the opposite.
When the steric bulk signifies that the rotating substituents begin to intervene with one another, so-called “gearing” begins to happen the place the motions of the 2 develop into coupled by the gears. The rotations are actually a concerted asynchronous course of.
So now to my concluding thought. The above is a straightforward instance of gearing involving rotation about two coupled bonds. So what number of bonds will be concurrently geared in order that when one rotates, the others do as properly? I’m now trying to find an instance of three such bonds geared collectively. And is there a restrict to what number of can achieve this in live performance? Right here we enter into analogy with bond cleavage, the place there are quite a few examples of bonds breaking in live performance, if not in synchrony. Most pericyclic processes are of this sort. Is there an identical patten in bond rotations?
[ad_2]