
[ad_1]
The schematic illustration of a chemical response mechanism is usually drawn utilizing a palette of arrows connecting or annotating the assorted molecular constructions concerned. These could be chosen from a chemical arrows palette, taken for this objective from the generally used construction drawing program Chemdraw. Explanations of methods to apply the person arrows should not at all times simple to search out nevertheless! Circled in pink are those to be mentioned right here, though most carry fascinating and sometimes refined meanings!‡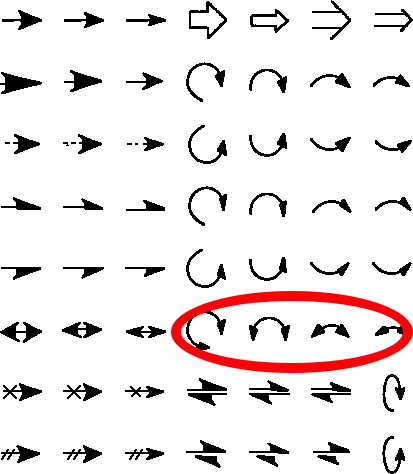
The commonest that means of the double-headed arrow might be finest illustrated by the scheme under, which includes the addition of a nucleophile to a carbonyl compound, forming a presumed “tetrahedral” intermediate, which is then instantly adopted by the eviction of a leaving group – the chloride anion within the instance under. The 2 pink arrows present an electron pair firstly shifting to the oxygen, after which with the reverse arrow 2 again to reform the carbonyl group. This course of known as an addition/elimination mechanism. It’s due to this fact tempting to conflate the 2 steps into one by as an alternative utilizing a double-headed arrow (3, blue), which if nothing else, saves a little bit little bit of time within the drawing – a helpful examination method!
After all, the highest scheme (pink arrows) is a two-step course of, involving a discrete (tetrahedral) intermediate and two transition states. The conflated scheme under it (blue arrows) may indicate (or not) a single-step course of with a single transition state. Since few individuals who draw such schemes have any data on whether or not it’s a two-step or a single step course of, the precise chemical that means of the double-headed arrow is left implicitly ambiguous, with out implying something about what number of discrete steps are concerned. Nevertheless, it’s tempting to conclude that the primary pink arrow (1) reduces the double bond order of the carbonyl group to a single bond, which could due to this fact be anticipated to elongate and the second pink arrow (2) reforms the double bond, thus shortening the bond. The 2 arrows clearly don’t transfer concurrently. The conflated third arrow (3) leaves the standing of the carbonyl bond size modifications undefined, or may it imply that it first will get longer after which shorter alongside the response path, relying in fact on which strikes first!
Enter computation, the place the vitality pathway of such a response could be computed, together with geometries at every stage. Right here I discover three examples† to see what outcomes (ωB97XD/De2-TZVPP/SCRF=DCM), FAIR DOI: 10.14469/hpc/13171.
Acetyl chloride + Methanol.
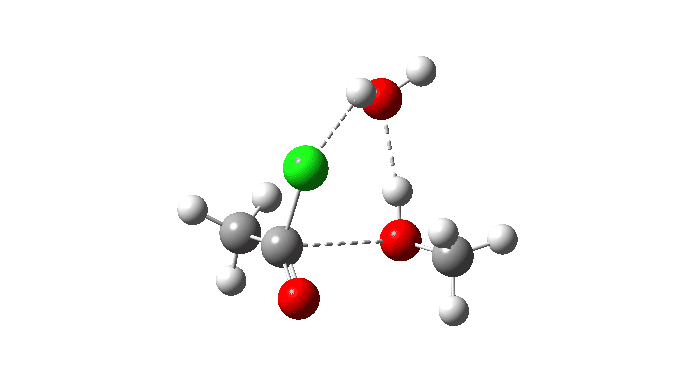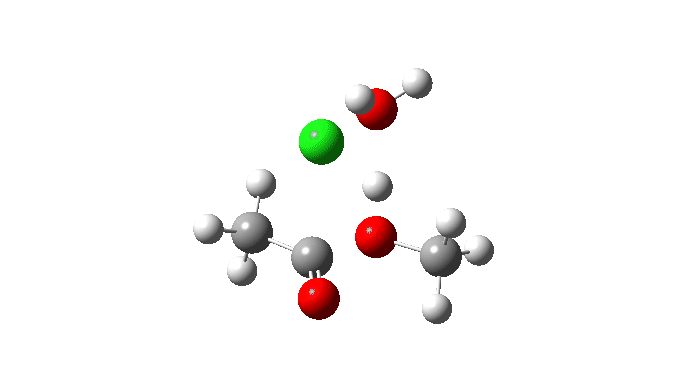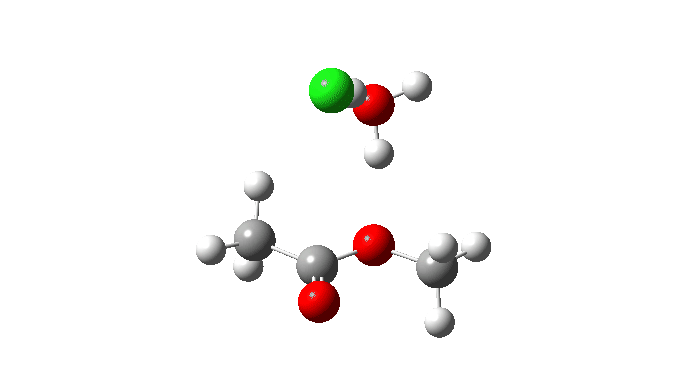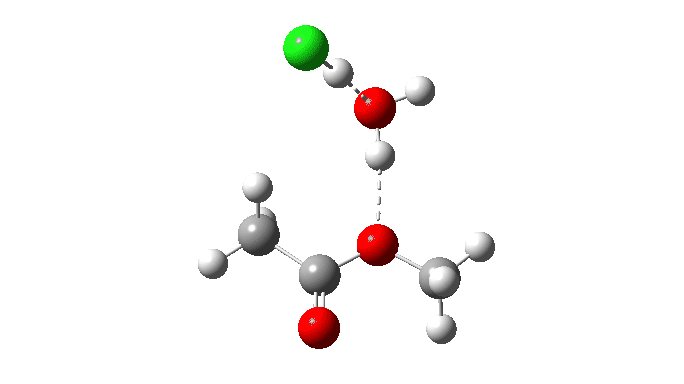
This makes use of a mannequin by which a proton switch from the methanol to the chloride anion is facilitated by water. This allows (however doesn’t implement) a steady concerted course of to happen. This emerges from the computed intrinsic response coordinate (IRC) as having a low barrier and an exothermic response, which agrees with experimental remark. The required proton switch is a part of the concerted course of, albeit occurring in a second decrease vitality stage (IRC ~+1.5).
However check out how the carbonyl bond size modifications alongside this IRC. It first shortens, and solely begins to elongate because the chloride is evicted. So the carbonyl group really contracts in size on the transition state, the other of what could be inferred by utilizing a double-headed arrow.
Additionally included is the dipole second response, which does appear to correspond to the formation of an ionic intermediate!
Acetyl chloride + HF.
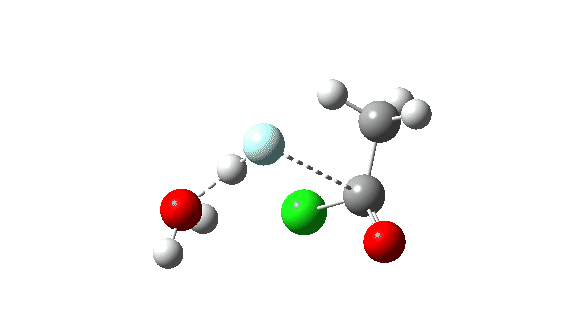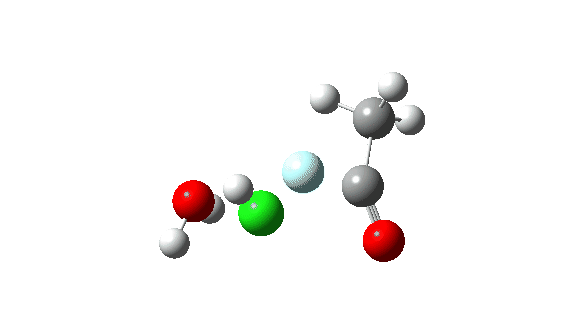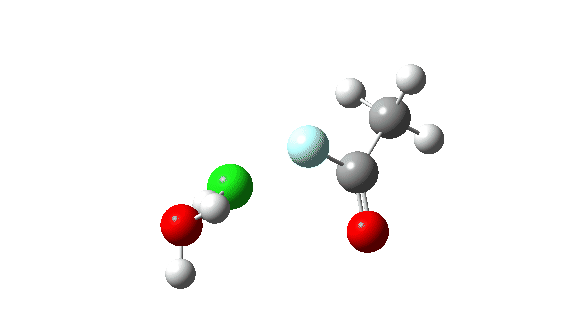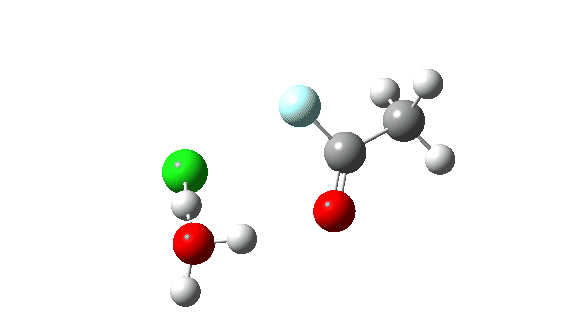
Hydrogen fluoride as a nucleophile changing methanol reveals a a lot larger barrier, since it’s much less good as a nucleophile on this context.
Once more, observe the bond size response of the carbonyl group, which is at its shortest on the (single step) transition state.
This corresponds to a unique interpretation of the double-headed arrow, as per under, however occuring as a part of a single concerted course of not involving any intermediate.
The dipole second response is moderately totally different from methanol.
Acetyl chloride + Methylamine.
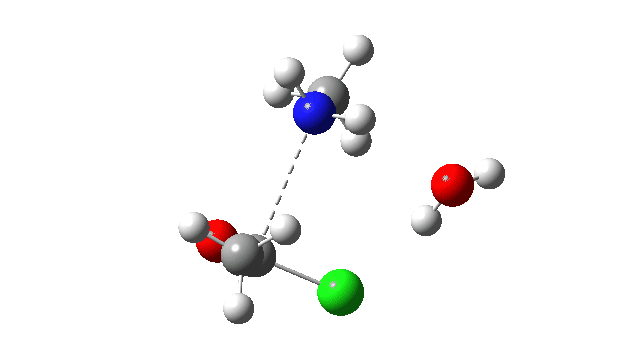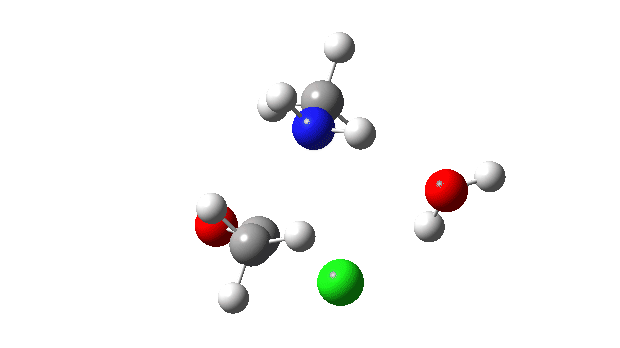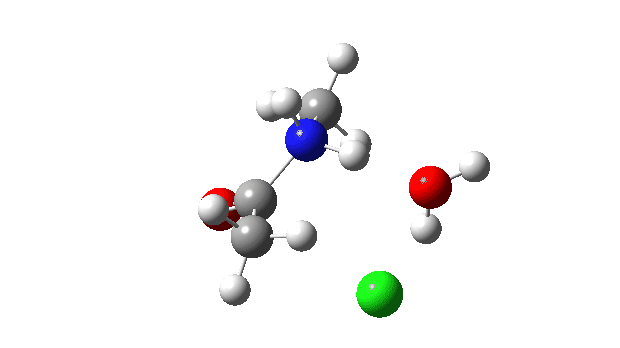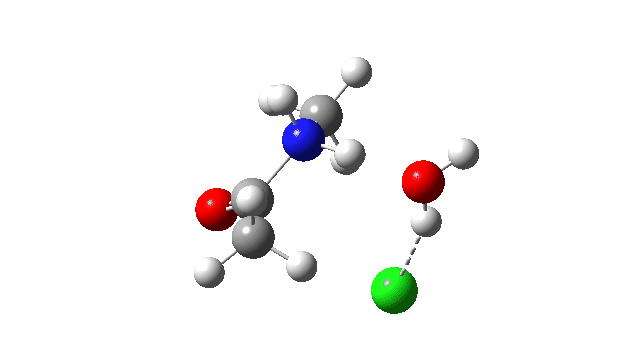
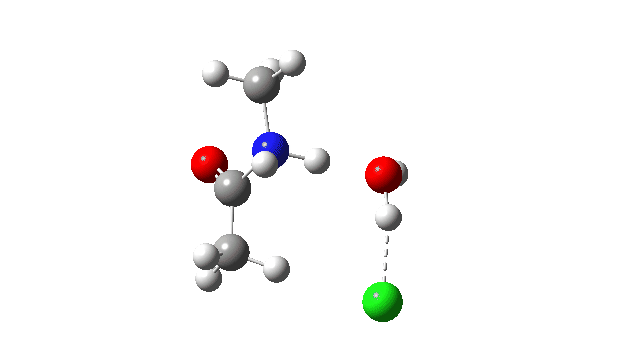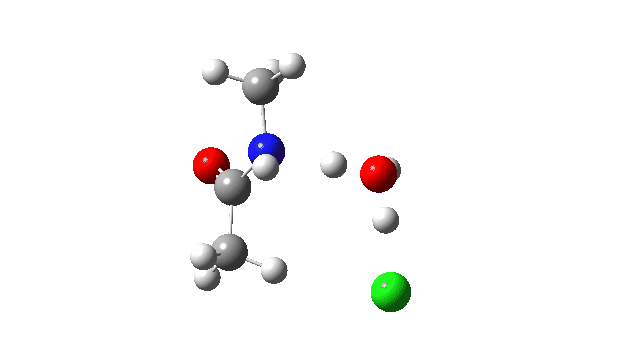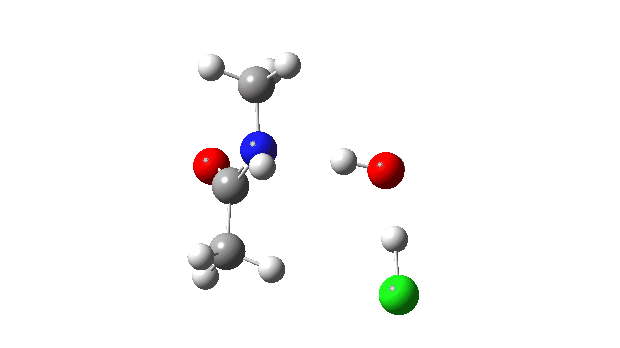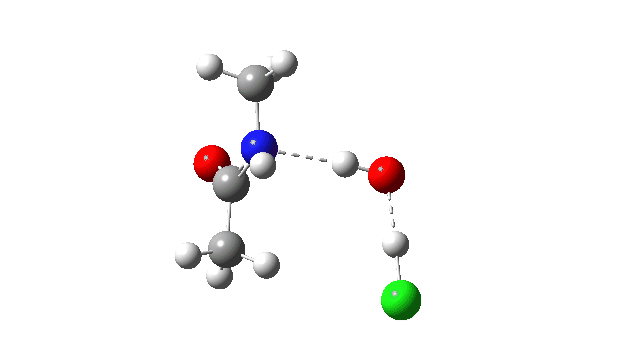
The vitality profile now reveals two distinct transition states (IRC ~7 and once more at 0.0). The primary is a really low vitality addition to the carbonyl group with concerted eviction of the chloride anion, which solely hydrogen bonds to the water proven. The second stage is the proton switch from the nitrogen to the water and thence relayed to the chloride anion, for which a transition state at IRC ~0.0 is discovered.
However now observe the bond size response, which reveals a definite most across the first transition state (IRC ~7). That is the other behaviour to the earlier two programs, and now certainly matches the unique inferences one may make from the double headed arrow.
So we are able to conclude that there are in actual fact TWO varieties of double-headed arrow which might be utilized in mechanistic representations. The primary is when arrow 1 is forward of arrow 2 (pink), leading to preliminary weakening of the carbonyl bond. The second is when arrow 4 is forward of arrow 5, leading to preliminary strengthening of the carbonyl bond.
Maybe to keep away from confusion, we actually want two totally different representations of a double-headed arrow to obviously differentiate them! Maybe a reversal of the route of the arrowhead? However that doesn’t (but?) exist within the Chemdraw palette.
‡That is a part of the arcane “information” of chemistry which is usually absorbed moderately than learnt by college students of the topic, however which in consequence turns into a language that turns into inscrutable to anybody else! †One other instance was famous within the earlier publish.
[ad_2]