
[ad_1]
Core Ideas
Aqueous options are one of the crucial necessary varieties of combination inside and out of doors the lab. Try this text to be taught extra concerning the traits and properties of aqueous options!
Subjects Coated in Different Articles
What are Aqueous Options?
Chemists use the time period “aqueous” to explain an answer with water as its solvent. Because of the distinctive chemistry of water, aqueous options have sure properties not shared with options that use different solvents frequent to the chemistry lab, equivalent to hexane, acetone, or dichloromethane.
Specifically, water is each polar and protic. As a polar solvent, water has a polar construction that permits the solubilization, or molecular mixing, of polar molecules equivalent to alcohols and amines in aqueous options. Conversely, nonpolar molecules equivalent to hydrocarbons typically can not develop into solubilized into aqueous options. This follows the “like dissolves like” mnemonic. As a protic solvent, water is able to forming hydrogen bonds, one of many strongest intermolecular forces. The protic nature of water permits many salts to dissolve into aqueous options, as a result of formation of sturdy ion-dipole interactions. Nonetheless, whereas ionic compounds are likely to dissolve extra simply in aqueous options than different solvent techniques, not all salts are soluble in water.
These properties of water permit chemists to make use of aqueous options in lab for response workups. Specifically, water permits the separation of water-soluble, or hydrophilic, substances from natural solvents. Exterior the lab, aqueous options are in every single place! The water in oceans and rivers counts as aqueous answer, carrying a broad variety of salts and natural compounds to assist the organisms that inhabit them. Aqueous options, with a cleaning soap or detergent solute, are used to take away filth and germs from dishes, garments, surfaces, and human pores and skin. Any drink, from tapwater, milk, espresso, or soda, will be considered an aqueous answer carrying sugar, carbon dioxide, salts, and different vitamins into the human physique, which itself has aqueous options equivalent to blood, urine, and sweat.
Composition of Aqueous Options
Chemists outline a answer as a system with two fundamental elements, the solvent and the solute. The solvent is the liquid section of the system; as talked about earlier than, aqueous options have water because the solvent. The solute is the substance suspended within the solvent; as talked about earlier than, aqueous options sometimes contain polar molecules and salts.
Ionic vs Molecular Solutes
When in suspension of water, solutes with an ionic bond, referred to as ionic solutes, behave totally different than wholly covalent solutes, additionally referred to as molecular solutes. Specifically, ionic solutes dissociate into their respective anion and cation when dissolved in water. For instance, once you submerge desk salt, NaCl, into water, it separates into positively charged sodium ions and negatively charged chloride ions. This dissolution occurs as a result of the ions in NaCl have extra attraction to the water solvent than one another. Particularly, the Na+ ions develop into interested in the adverse dipoles of water, whereas Cl– have attraction to the optimistic water dipoles.
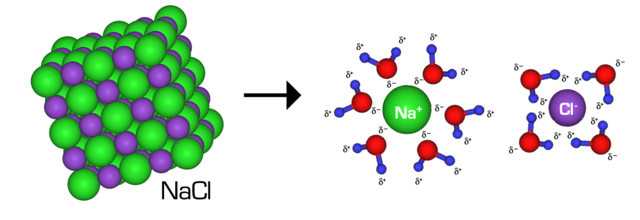
Molecular solutes, like glucose, lack an simply breakable ionic bond, in order that they develop into solubilized in water via a unique mechanism. In strong kind, glucose molecules appeal to one another and kind a crystalline matrix. When submerged in an aqueous answer, glucose molecules separate from the matrix and develop into surrounded by water molecules. This technique of changing into surrounded by water is known as solvation. In glucose, solvation entails the formation of hydrogen bonds and dipole bonds with the water molecules. Importantly, in contrast to ionic solutes, molecular solutes don’t break down into part items, however fairly preserve their molecular buildings.

Electrolytes in Aqueous Options
In aqueous answer, salts and different ionic solutes can conduct electrical energy. This is because of the truth that dissolving ionic compounds introduces conductive charged particles into the answer. Due to this property, chemists typically use the time period “electrolyte” to seek advice from dissociated ions in water. Conducting electrical energy is a vital property for the aqueous options concerned in batteries.
Nonelectrolytes
Solutes not able to conducting electrical energy go by the title nonelectrolytes. This contains molecular solutes that don’t ionically dissociate, in addition to intact ionic molecules. Which means ionic solutes like silver nitrate are nonelectrolytes, however develop into electrolytes when dissolved into separate ions.

Robust vs Weak Electrolytes
Chemists prefer to classify electrolytes as “sturdy” or “weak” relying on how properly they conduct electrical energy. Robust electrolytes are extremely conductive, which means that they switch a excessive proportion of electrical present that passes via its aqueous answer. Weak electrolytes, against this, switch a decrease proportion electrical energy.
The conductivity, and thus power, of an electrolyte is determined by the diploma that it dissociates in water. An ionic compound that’s extremely soluble in water, equivalent to ammonium nitrate, will dissociate nearly fully in an aqueous answer, making it a robust electrolyte. A much less soluble compound, like sodium bicarbonate, doesn’t utterly dissociate in water, and a excessive proportion of NaHCO3 stays intact, which finally makes it a weak electrolyte.

This idea is just like strong-weak acid idea, and actually, all sturdy acids are sturdy electrolytes whereas all weak acids are weak electrolytes.
[ad_2]