
[ad_1]
Core Ideas
On this article, we discover the electrochemistry behind batteries. We look at widespread examples together with major batteries, zinc-copper batteries, lead-acid batteries, nickel-cadmium batteries, and gasoline cells.
Matters Coated in Different Articles
Redox: The Chemistry behind Batteries
Although they could appear high-tech, batteries work based on pretty primary physics and chemistry. Particularly, you may clarify the exercise of a battery in molecular phrases, as vessels for a chemical response that ends in an electrical present. On the chemical degree, this present is a stream of electrons. Chemists decribe reactions that switch electrons, together with people who happen in batteries, as “redox reactions“.
In battery redox reactions, atoms of 1 metallic ingredient oxidize, or lose electrons, whereas atoms of one other metallic cut back, or achieve electrons, which in the end produces the specified electron present. They do that as a result of the 2 metals have completely different discount potentials, that means they’ll spontaneously switch electrons when in touch. Particularly, the metallic with the decrease potential oxidizes, and is thus known as the anode, whereas the upper potential metallic reduces, which chemists name the cathode. College students normally keep in mind this terminology via the mnemonic “RED CAT AN OX” which implies “REDuction on the CAThode whereas the ANode OXidizes”.
Let’s check out this terminology in motion and contemplate the first battery.
Major Batteries
Commericially out there batteries might be divided into two classes: major and secondary batteries. Major batteries are single use and disposable, which incorporates the Double-A and Triple-A varieties we purchase for widespread family electronics, like flashlights and distant controls.

As we are able to see within the above diagram, electrons stream from the high-potential anode to the low-potential cathode via a wire that connects the 2 metals. Most frequently, the cathode accommodates manganese whereas the anode accommodates zinc. The electrolyte is a paste containing water, starch, ammonium chloride (NH4Cl), and insoluble manganese (IV) oxide (MnO2). The electrolyte serves to offer manganese cations for discount, in addition to ammonia and chloride to precipitate oxidized zinc cations into insoluble Zn(NH3)2Cl2. This insoluble zinc complicated can’t be simply lowered again into impartial Zn, indicating that this response is in the end irreversible. Thus, major batteries can’t recharge. Regardless of this, major batteries would not have a excessive price to fabricate, making them a enough selection for widespread electronics.
Secondary Batteries
Secondary batteries, in contrast, are rechargeable. For an instance of a secondary battery, contemplate the Cu/Zn galvanic cell:

The Cu/Zn galvanic cell incorporates a copper cathode and a zinc anode. Each electrodes have a surrounding answer with cationic types of every metallic, within the type of metallic sulfate. The salt bridge of the cell, normally involving a easy salt like NaCl ot KCl, permits charged electrolytes to stream into every electrode. As one answer loses and the opposite good points electrons, these electrolytes preserve an electrochemical stability that permits the redox chemistry to happen. Because the galvanic cell runs, the strong zinc atoms will oxidize into aqueous Zn2+, which transfers electrons to the copper answer, decreasing the Cu2+ cations to strong copper that deposits on the copper electrode. This exercise happens based on the next response equations:
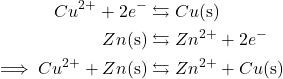
Importantly, in contrast to major batteries, the redox reactions concerned within the exercise of galvanic cells might be reversed with the appliance of electrical present. This present briefly converts the galvanic cell into an electrolytic cell, which oxidizes the copper again into Cu2+ and reduces Zn2+ into impartial zinc. Thus, secondary batteries can recharge. Due to this spectacular means, secondary batteries are utilized in a wide range of helpful applied sciences, from cellphones to vehicles to energy instruments. Let’s check out a number of vital examples.
Lead-Acid Batteries
One instance of a typical secondary battery is the lead-acid battery. Invented in 1859, the lead-acid battery is understood for being cheap to supply whereas providing excessive output when initiated. For that reason, lead-acid batteries exist in just about all commercially out there vehicles for engine ignition.

Lead-acid batteries contain sulfuric acid answer with two submerged metallic plates, a impartial lead anode and a lead (IV) oxide cathode (PbO2). Because the battery initiates, the acidic medium helps to oxidize the impartial lead into lead (II) sulfate (PbSO4). Concurrently, the lead oxide on the cathode additionally converts into PbSO4, however via discount. The redox exercise of lead-acid batteries might be summarized via the next response equations:
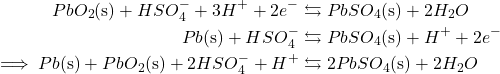
Importantly, the PbSO4 product is strong, which implies that it precipitates onto the electrodes because the response progresses. With an added electrical present, the PbSO4 merely reconverts into elemental lead and lead (IV) oxide, permitting recharge.
Nickel-Cadmium Batteries
The nickel-cadmium (Ni/Cd or NiCad) battery offers one other instance of a secondary battery. Light-weight and rechargeable, Ni/Cd batteries have use in a wide range of electrical home equipment and energy instruments.
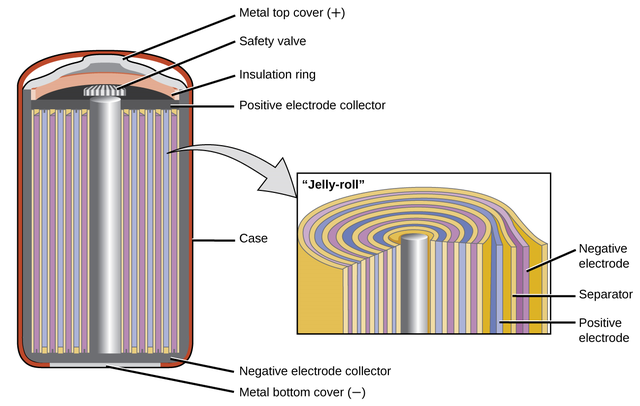
Ni/Cd batteries implement a “jelly roll” construction the place metallic layers of cadmium anodes and nickel cathodes are separated by a polymer with aqueous alkaline answer (the separators). These separators enable shut positioning of the electrodes, which reduces inside resistance for environment friendly electron discharge. The nickel cathodes are literally nickel (III) oxo-hydroxide (NiO(OH)), which reduces into nickel (II) hydroxide (Ni(OH)2). Equally, the cadmium transforms into its respective hydroxide, cadmium (II) hydroxide (Cd(OH)2), however via discount. The redox exercise of Ni/Cd batteries might be summarized via the next response equations:

Just like the lead-acid battery, the hydroxide merchandise turn into a strong precipitate on the floor of the electrodes. With utility of a recharging present, the redox reactions reverse which restores the capability of the battery.
Gasoline Cell Batteries
Gasoline cell batteries are a promising expertise that gives an influence supply with out fossil gasoline enter nor poisonous heavy metals. In few phrases, gasoline cells operate like a galvanic cell that requires fixed inputs of hydrogen and oxygen fuel, with an output of gaseous water. Due to this use of exterior gasoline fuel, gasoline cells don’t rely as major nor secondary batteries. The next diagram summarizes the redox exercise of gasoline cells.

Briefly, the central electrolyte catalyzes the paired conversion of hydrogen fuel into protons (H+) and oxygen fuel into oxide (O2-), producing water. This exercise might be summarized within the following redox reactions:
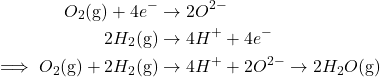
Whereas the expertise of gasoline cells appears thrilling, present gasoline cells stay comparatively inefficient. This is because of the truth that the discount of oxygen to oxide stays sluggish. Present analysis goals to discover a appropriate catalyst to hurry up this discount. One promising analysis lead entails the usage of a platinum catalyst. Hopefully, with additional examine, environment friendly, clear gasoline cells will present a brand new supply of vitality to assist in the inexperienced vitality transition.
[ad_2]