
[ad_1]
Respiratory pigments are metalloproteins that transport O2, one of the best identified being the brilliant pink/crimson colored hemoglobin in human blood. The color derives from Fe2+ on the core of a tetraporphyrin ring. However much less well-known is blue blood, and right here the color derives from an oxyhemocyanin unit primarily based on Cu1+ (the de-oxy type is colourless) reasonably than iron. See under for the carapace of a pink rock crab.
 Right here I check out this very uncommon construction, the core of which is an imidazole ring coordinated by way of nitrogen to the steel Cu.
Right here I check out this very uncommon construction, the core of which is an imidazole ring coordinated by way of nitrogen to the steel Cu. A search of the crystal construction database for the next sub-structure
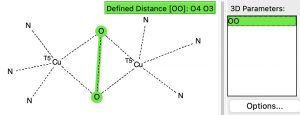
reveals 12 hits, with a variety of O-O distances starting from 1.37 to 1.54Å. A histogram of the O-O lengths within the Cu(O-O)Cu sub construction proven above exhibits fairly a distribution amongst the 12 identified examples.
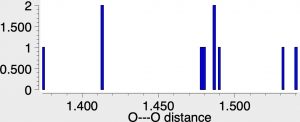
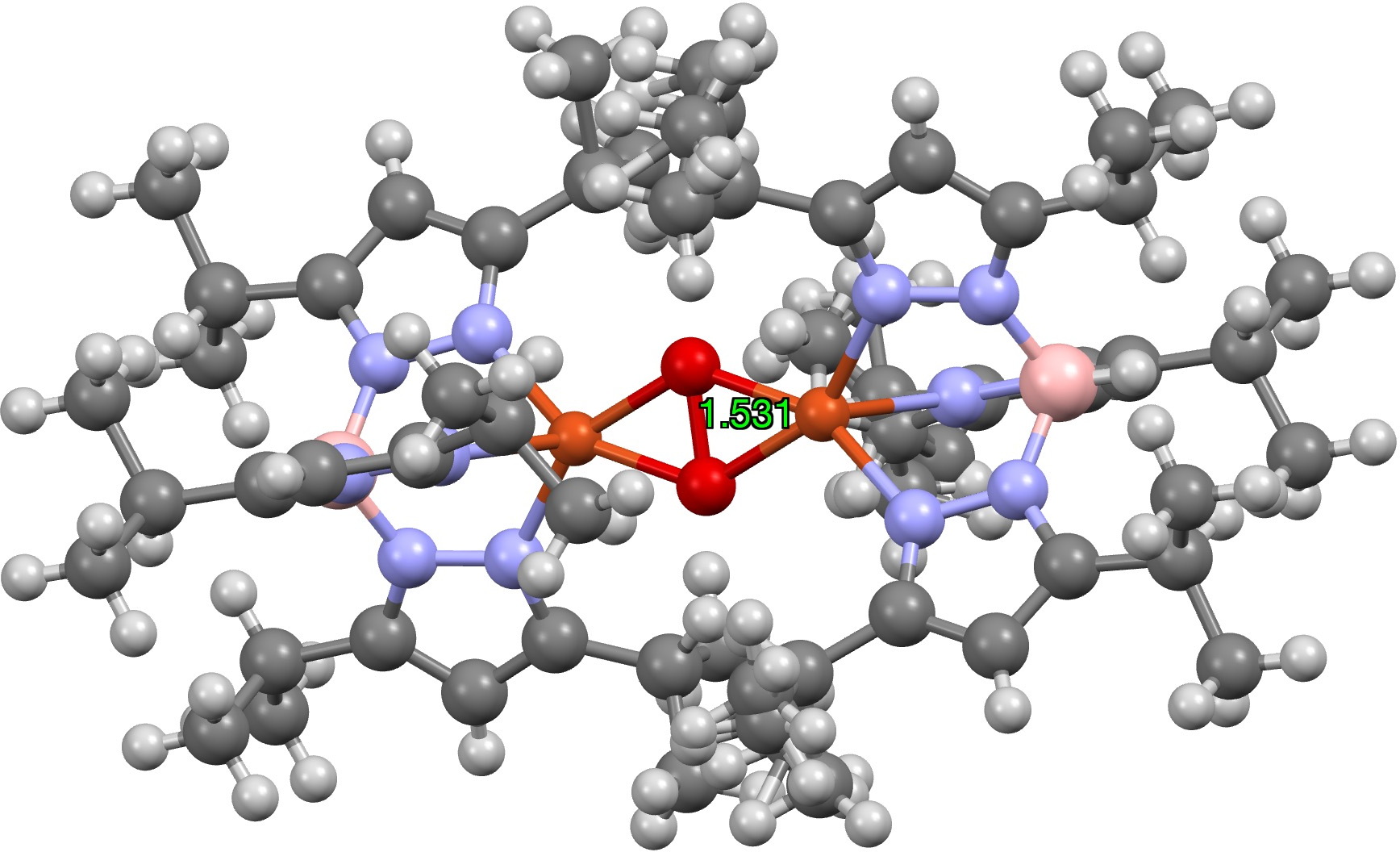
Of those, one (UTETEU[1], DOI: [2]) is probably the closest to the oxyhemocyanin core, albeit with the imidazole heterocycle changed by the isomeric pyrazole ring (no Ag or Au examples are identified). The general 2+ cost deriving from two Cu1+ models is internally balanced with two 4-coordinate B1- finish caps, and this method was chosen because the beginning mannequin for some computational research.[3]
Firstly, the crystal construction reveals an O-O distance of 1.531Å; the O=O distance (from crystal buildings the place it’s current) is ~1.24Å (DOI: 10.5517/cct597h) for impartial (triplet?) oxygen, ~1.50Å for the dianion O22- and 1.32Å for the monoanion O21-[4].

Computational fashions had been constructed on the ωB97XD/Def2-SVPP degree, FAIR Information DOI: 10.14469/hpc/12584.
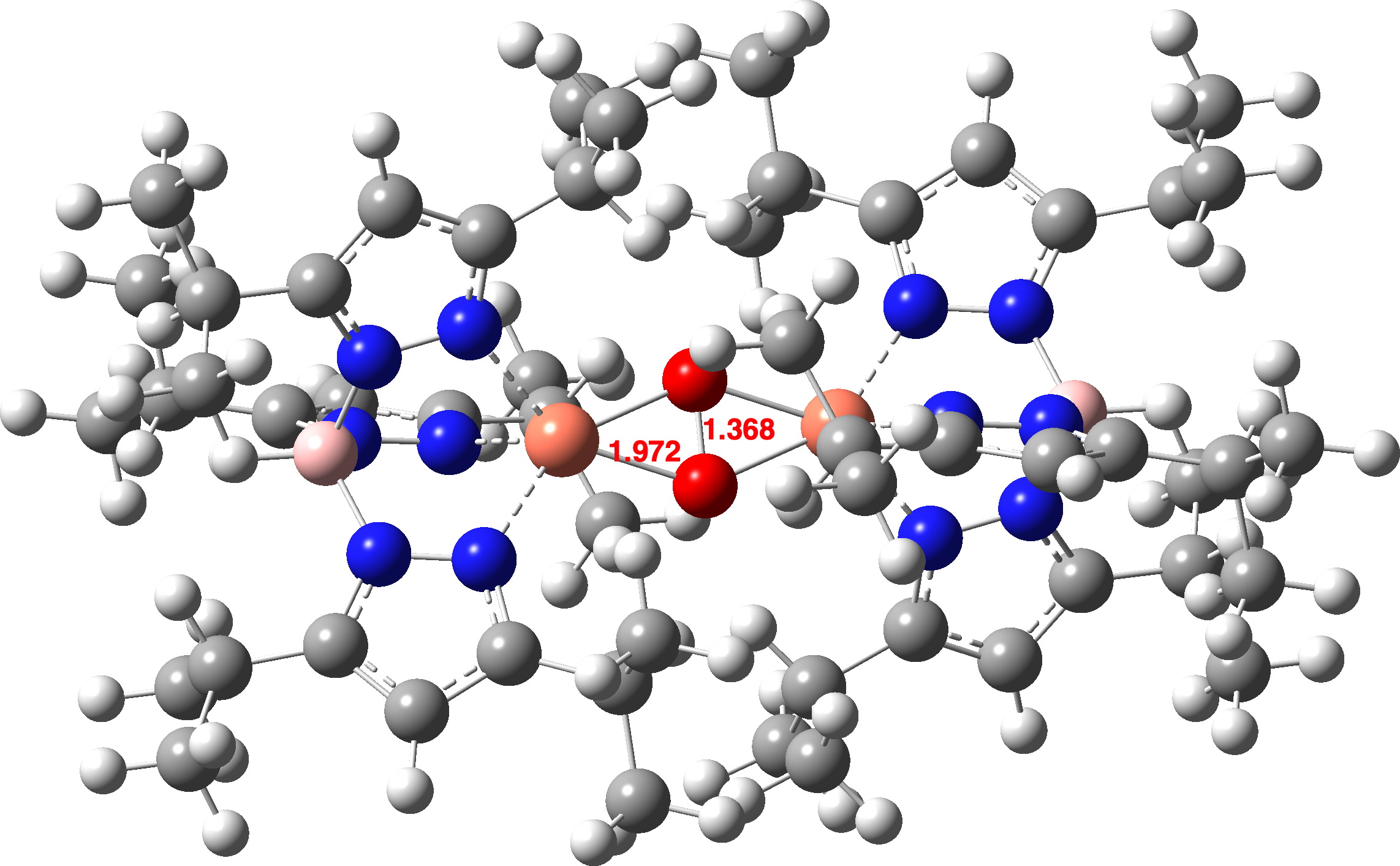
The computed O-O distance for a singlet state of the advanced is shorter than that measured within the crystal construction (1.368 vs 1.531Å). On the higher Def2-TZVPP foundation set degree, the O-O bond size is 1.379Å, nonetheless shorter. A mannequin of singlet state oxyhemocyanin itself (Def2-TZVPP) as a di-cation (these fees are balanced by carboxylate anions from the encircling protein) exhibits a really related O-O bond size (1.361Å).
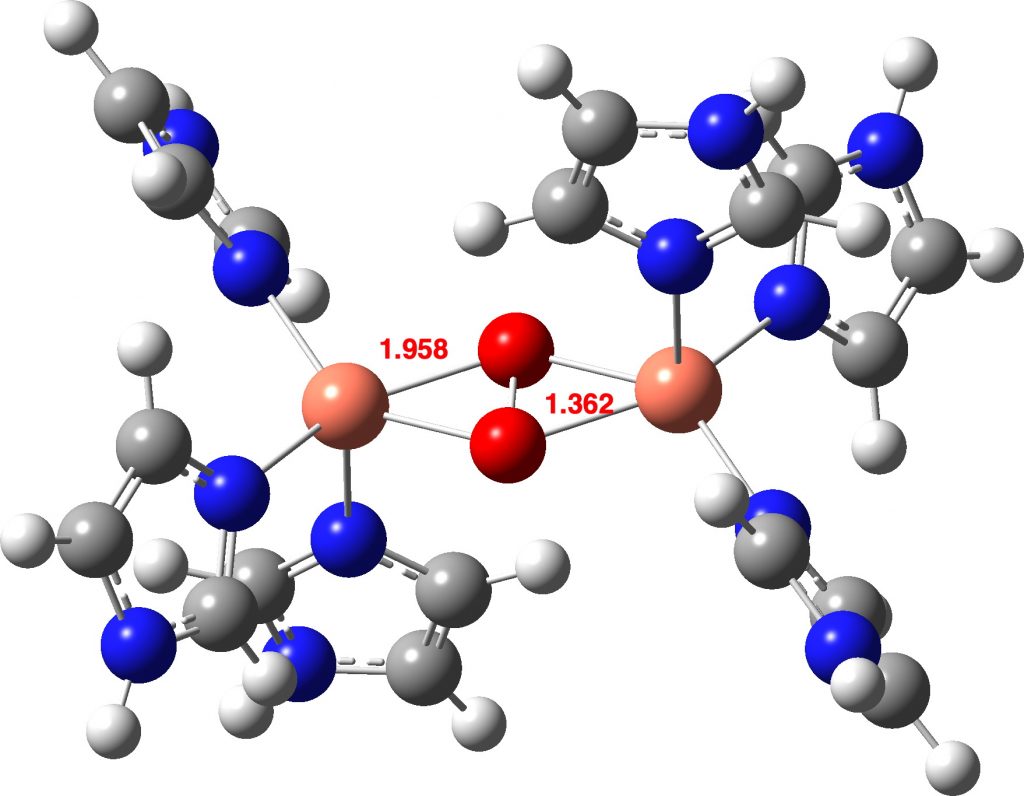
How concerning the oxyhemocyanin as a triplet state, the identical state of remoted oxygen itself? Oxyhemocyanin now has a O-O distance of 1.477Å (Def2-TZVPP) and a Cu-O distance of 1.972 (1.934 from crystal construction of UTETEU).

So we could conclude from this transient investigation into the buildings of this element of “blue blood” captures oxygen as a sandwich between two copper atoms (a mode very in contrast to the iron equal in hemoglobin), and furthermore that the spin state on this seize retains the triplet motif of gaseous oxygen itself.
[ad_2]