[ad_1]
Total construction of MslH
To analyze the catalytic mechanism of MslH, we carried out crystallographic research. Recombinant N-terminally His6-tagged MslH was expressed in Escherichia coli and purified for crystallization. The purified recombinant MslH migrated as a single band with a molecular weight of 48.5 kDa on SDS–PAGE, in good settlement with the calculated worth of 49.4 kDa (Supplementary Fig. 1a). In distinction, a gel-filtration experiment indicated a molecular weight of 410 kDa, suggesting that the recombinant MslH is an octameric enzyme (Supplementary Fig. 2). The X-ray crystal construction of MslH:apo, listed within the I422 area group with one monomer within the uneven unit, was solved at 2.30 Å decision. The MslH:apo construction was initially decided by the sulfur single-wavelength anomalous diffraction (S-SAD) technique, for the reason that molecular substitute (MR) makes an attempt utilizing the templates recommended by the Phyre2 evaluation19 as search fashions have been unsuccessful (Supplementary Fig. 3a and Desk 1). Consequently, a recombinant N-terminally His6-tagged full-length MslA was ready by co-expression with MslB1 in E. coli, since MslA expressed in E. coli was solely soluble when complexed with MslB119. Nevertheless, the advanced construction with MslH and full-length MslA couldn’t be obtained by co-crystallization and soaking experiments, maybe as a result of response on wild-type MslA. Therefore, recombinant N-terminally His6-tagged MslAΔTrp21, which lacked the C-terminal Trp21, and MslA Trp21G variants, with an achiral glycine as an alternative of Trp21, have been ready by co-expression with MslB1 in E. coli. The co-crystallization was completed by mixing MslH:MslAΔTrp21-MslB1 or MslH:MslA Trp21G-MslB1 in a 1:1.2 mole ratio. Consequently, the X-ray crystal constructions of the MslH:MslAΔTrp21 and MslH:MslA Trp21G complexes at 2.29 Å and a couple of.12 Å resolutions, respectively, with one monomer within the uneven unit, that are the identical because the MslH:apo construction, have been efficiently obtained (Supplementary Desk 1). Each crystal constructions have been solved by the MR technique, utilizing the MslH:apo construction because the search mannequin (Fig. 2), and the enzyme fashioned the same biologically energetic octamer to that of the MslH:apo construction (Fig. 3 and Supplementary Fig. 4). The MslH constructions complexed with MslA analogues adopted nearly the identical conformations because the MslH:apo construction, with Cα Root Imply Sq. Deviations (RMSDs) of 0.17 Å (MslH:apo vs. MslH:MslAΔTrp21) and 0.16 Å (MslH:apo vs. MslH:MslA Trp21G) (Supplementary Fig. 3b, c).
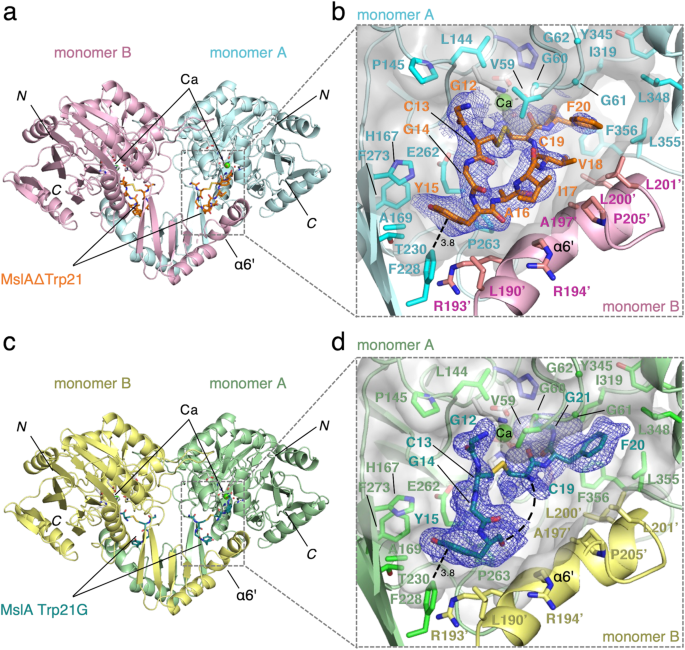
Overview of the dimeric MslH:MslAΔTrp21 construction (a) and the MslH:MslA Trp21G construction (c). Shut-up view of the MslH-MslA analogue binding cleft with the floor mannequin within the MslH:MslAΔTrp21 construction (b) and that of the MslH:MslA Trp21G construction (d). A consultant OMIT electron density map (mFo-DFc) contoured to 2σ across the MslA analogue is proven by the blue-colored mesh. The straight dashed traces characterize the distances in Å. The curved dashed line represents the lacking MslA Ala16-Val18 residues.
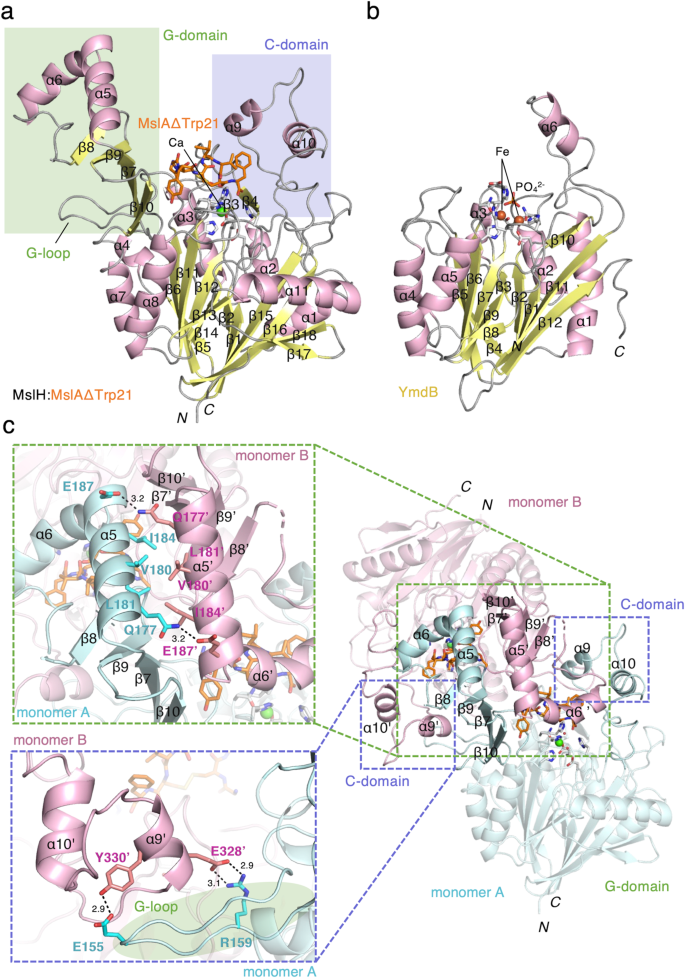
Stereographic ribbon illustrations of the MslH:MslAΔTrp21 monomer construction (a) and the reported YmdB monomer construction (PDB ID: 4B2O) (b)26. α-Helices and β-sheets are coloured pink and yellow, respectively. The secondary structural components are labeled in accordance with every topology. The residues and steel ions within the active-site surroundings are proven as sticks coloured by atom sort and spheres, respectively. The carbon-backbone of MslAΔTrp21 is coloured orange. c Overview of dimer formation targeted on the C-domain and G-domain.
Every monomer consists of a giant area with a grip-like subdomain (G-domain: Ala141-Pro234) and a cover-like subdomain (C-domain: Ser317-Trp363) (Fig. 3a and Supplementary Figs. 5 and 6a). Of the eleven α-helices and 18 β-sheets, six α-helices and twelve β-sheets comprise the big area. Amongst them, six β-sheets (β1, β2, and β15–β18) and 6 β-sheets (β5, β6, and β11–β14) face one another on the middle of the big area, to type a blended parallel and antiparallel β-strand construction, surrounded by α-helices (α1–3, α7, α8, and α11) to create the αββα barrel structure (Fig. 3a). Of the remaining α-helices and β-sheets, two α-helices (α9 and α10) take part within the development of the C-domain. This area comprises a number of lengthy loops and partially covers one facet of the highest of the αββα structure within the massive area. The G-domain consists of two α-helices (α5 and α6) and 4 antiparallel β-sheets (β7–β10), and is connected to nearly the other web site of the C-domain on the highest of the αββα structure. Two α-helices (α5 and α6) of the G-domain on the monomer protrude towards one other dimer-forming monomer, and take part within the dimer formation along with a part of the outer floor of the αββα core construction (Fig. 3c). As well as, the G-domain is encapsulated by the C-domain of the opposite monomer by way of a number of contacts, comparable to α9’ of the C-domain within the different monomer with the lengthy loop construction between β6 and β7 of the G-domain (G-loop) (Fig. 3a, c). Consequently, α6’ from one other monomer, along with the 4 antiparallel β-sheets on the G-domain and a part of the C-domain of the monomer, varieties a big cleft on the sting of the αββα structure within the core construction (Fig. 2).
Construction-based similarity searches revealed that MslH possesses a calcineurin-like fold25 as a part of its construction, as beforehand predicted by a Phyre2 evaluation19. The general construction of the MslH:apo construction is most just like that of the metal-dependent phosphodiesterase YmdB in Bacillus subtilis (PDB ID: 4B2O)26, with an RMSD worth of two.26 Å for 71 Cα atoms (equivalent to 16% of the amino acids of MslH), though their amino acid sequence identification is barely 16%. The structural similarity is principally noticed within the αββα structure (Fig. 3b and Supplementary Figs. 6 and 7). A structural comparability of each proteins revealed that the 2 β-strands (β17 and β18) and one α-helix (α11) constructions on the C-terminal facet have been attribute of MslH, among the many αββα architectures. Notable variations between the MslH and YmdB constructions have been noticed within the C-domain and G-domain in MslH (Fig. 3a, b and Supplementary Fig. 7). In these constructions, the C-domain in MslH is a steric homolog of the construction consisting of loops and α6 in YmdB, though that of MslH includes loops with two α-helices, α9 and α10. In distinction, the G-domain in MslH is absent in YmdB.
Lively-site structure of MslH
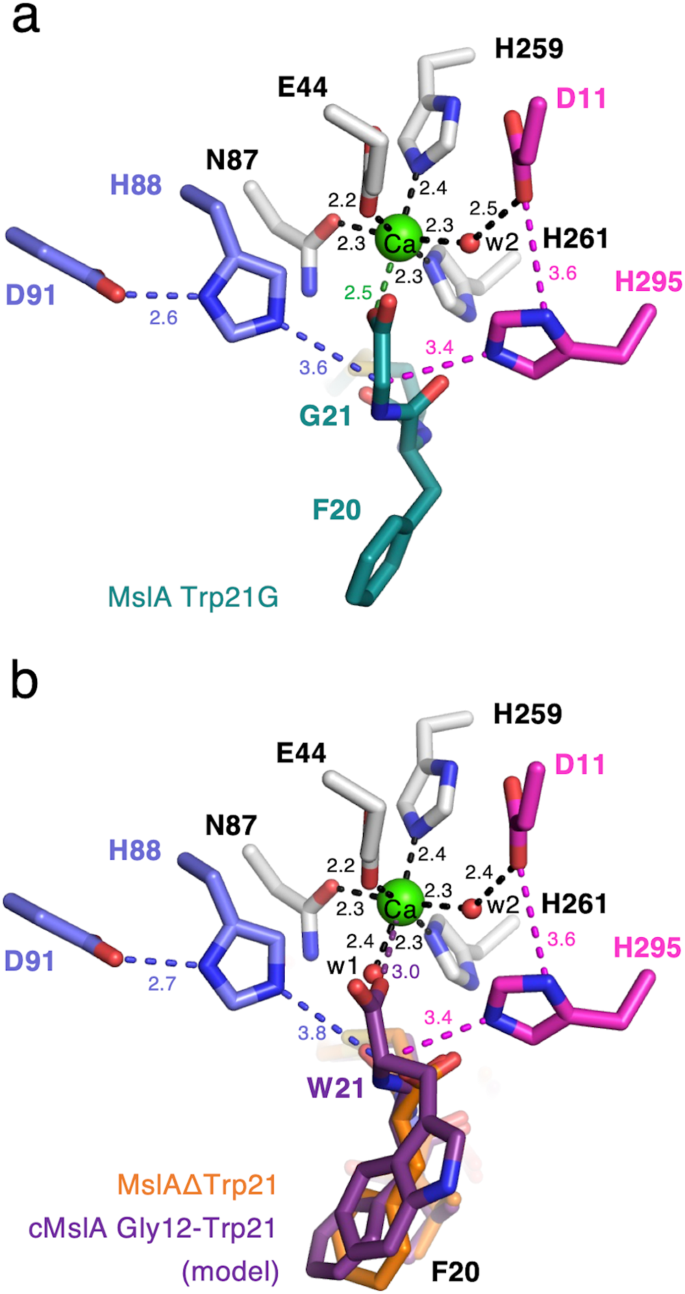
The electron density of MslAΔTrp21 within the MslH:MslAΔTrp21 crystal construction allowed us to find a cyclic peptide molecule (cMslA Gly12-Phe20), consisting of the Gly12-Phe20 half on the C-terminal facet of MslAΔTrp21 with a disulfide bond between Cys13 and Cys19, within the massive cleft conformed by two monomers (Fig. 2a, b). Nevertheless, no electron density equivalent to both the MslA chief peptide or MslB1 was recognized within the MslH:MslAΔTrp21 crystal construction. The cMslA Gly12-Phe20 of MslAΔTrp21 molecule is accommodated within the cleft with numerous interactions, comparable to a σ–π interplay between Phe228 of monomer A and Tyr15 in cMslA Gly12-Phe20 (3.8 Å), hydrophobic interactions between α6’ of monomer B and MslA Ala16-Val18, and polar interactions between Gly60 (3.4 Å)/His295 (2.7 Å) in MslH and the carboxyl group of the C-terminal MslA Phe20 of MslAΔTrp21 (Figs. 2b and 4a and Supplementary Fig. 8a). In a fashion just like the MslH:MslAΔTrp21 construction, the MslH:MslA Trp21G construction accommodated the MslA Trp21G molecule, consisting of the Gly12-Tyr15 and Cys19-Gly21 elements on the C-terminal facet of MslA Trp21G with a disulfide bond between Cys13 and Cys19, within the massive cleft (Fig. 2c, d and Supplementary Figs. 8b and 9a, b). As well as, the amide nitrogen and oxygen atoms between Phe20 and Gly21 in MslA Trp21G fashioned polar interactions with the oxygen atom within the spine of MslH Gly60 and the nitrogen atom of the His295 facet chain, respectively (2.6 Å and a couple of.5 Å) (Fig. 4b). As a result of the amide bond was acknowledged by MslH in the same method to the carboxyl group of the C-terminal MslA Phe20 (Supplementary Fig. 9c), the electron densities of MslA analogues doubtless represented the binding mode of the unique MslA substrate in MslH. Nevertheless, the disulfide bond noticed between Cys13 and Cys19 within the MslA analogues is just not current within the biosynthesized closing product, MS-271, which has disulfide linkages between Cys1-Cys13 and Cys7-Cys19 (Fig. 1). It’s hypothesized that in MS-271 maturation, the core peptide of MslA generates disulfide linkages between Cys1-Cys13 and Cys7-Cys19. To find out not directly whether or not the MslA analogues noticed within the MslH:MslAΔTrp21 and MslH:MslA Trp21G crystal constructions could be sure to the unique substrate-binding web site, we carried out in vitro assays with and with out DTT. Consequently, the addition of DTT to the MslH response had no impact on the MslH catalytic exercise (Supplementary Fig. 10). Moreover, even MslA variants with serine residues at Cys13 and Cys19 (MslA Cys13/19S), respectively, have been competent substrates within the MslH response, and exhibited the identical exercise as within the case of the intact MslA substrate (Supplementary Fig. 10). These observations counsel that the disulfide linkage between Cys13 and Cys19 in MslA analogues within the MslH constructions is just not obligatory for the catalytic exercise of MslH. Thus, the unique substrate would even be accepted in the identical cleft because the MslA analogues noticed within the MslH crystal constructions, and the catalytic response web site may happen round Gly21 on the MslA Trp21G molecule.
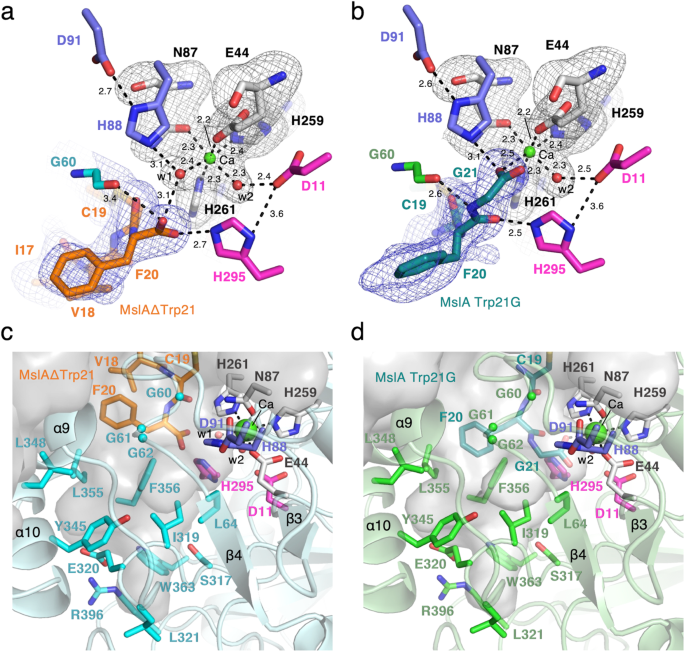
Shut-up view of the metal-coordination web site within the MslH:MslAΔTrp21 construction (a) and MslH:MslA Trp21G construction (b). A consultant OMIT electron density map (mFo-DFc) contoured to 2σ round MslA analogues is proven by the blue-colored mesh. One other consultant OMIT electron density map (mFo-DFc) contoured to 3σ round water molecules, metal-coordinated residues, and Ca(II) is proven by the gray-colored mesh. The dashed traces characterize the distances in Å. Floor illustration of the hydrophobic pocket across the C-terminus of MslA analogues within the MslH:MslAΔTrp21 construction (c) and MslH:MslA Trp21G construction (d).
The construction analyses additionally revealed that the C-terminal MslA Phe20 carboxyl group in MslAΔTrp21 and the C-terminal MslA Phe20 and Gly21 in MslA Trp21G are positioned close to a hydrophobic pocket consisting of MslH Gly60-62 and Leu64 on the core area and Ile319, Tyr345, Leu348, Leu355, and Phe356 on the C-domain in monomer A on the fringe of the cleft, respectively (Fig. 4c, d and Supplementary Figs. 8 and 9d). As well as, an structure that might assemble a six-coordination motif, consisting of 4 amino acid residues Glu44, Asn87, His259, and His261 and two water molecules (w1 and w2), was detected across the C-terminal carboxyl group of MslA Phe20 within the MslH:MslAΔTrp21 crystal construction (Fig. 4a and Supplementary Fig. 11a, b). This six-coordination motif is conserved within the MslH:apo and MslH:MslA Trp21G constructions (Supplementary Figs. 12 and 13), though one of many oxygen atoms of the C-terminal carboxyl group of MslA Gly21 occupied the area utilized by the w1 water molecule within the MslH:MslA Trp21G construction (Supplementary Figs. 9c and 13c), and is positioned on the entrance of the central ββ barrel behind the cleft. Within the six-coordination, the w2 water molecule hydrogen-bonds with the side-chain of Asp11 and is additional held within the construction by the steric contraction of the His295 side-chain, which varieties a hydrogen-bond with Asp11 (Fig. 4a, b). The opposite water (w1) within the MslH:MslAΔTrp21 construction and one of many oxygen atoms of the C-terminal carboxyl group of MslA Gly21 within the MslH:MslA Trp21G construction are positioned close to His88 forming a hydrogen-bond with Asp91, respectively (Fig. 4a, b). We additionally discovered some remaining electron density, which was clearly totally different from these of the peptides and water molecules, within the middle of the six-coordination system (Supplementary Fig. 14). When one other water molecule (w3) was added to the noticed electron density on the middle of the six-coordination web site, the Fo-Fc map remained optimistic across the added water molecule, indicating the presence of an unidentified steel ion as an alternative of a water molecule (Supplementary Fig. 14a), at a distance of two.5 Å from the oxygen atom of the Gly21 carboxyl group (Fig. 4b). These observations recommended that the catalytic middle liable for the epimerization response of MslH is on this area.
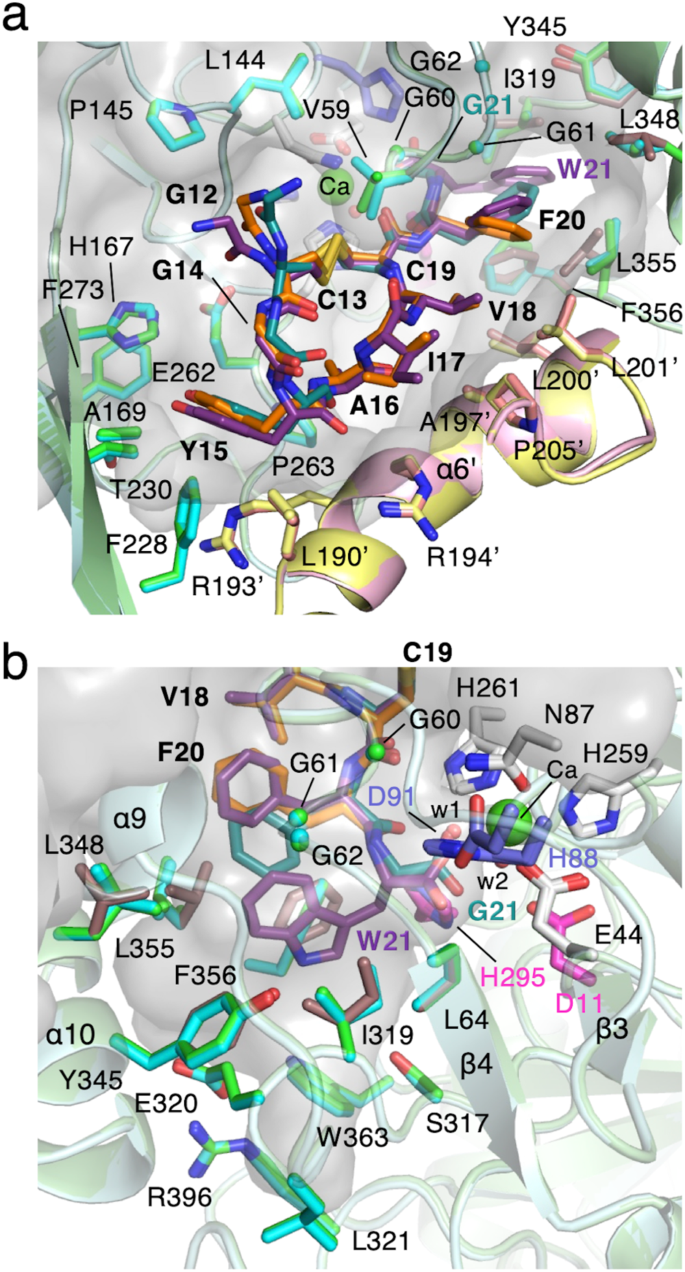
With a purpose to tackle this risk, we first carried out docking simulations with cMslA Gly12-Trp21, a Gly12-Trp21 peptide of MslA with a disulfide linkage between Cys13 and Cys19, to find out if this hydrophobic pocket is massive sufficient to accommodate the indole side-chain of the C-terminal residue Trp21 of wild-type MslA. The preliminary mannequin of cMslA Gly12-Trp21 was designed, primarily based on the cMslA Gly12-Phe20 structure within the MslH:MslAΔTrp21 construction. We chosen a number of amino acid residues contributing to the hydrophobic pocket flexibility, and ran AutoDock Vina docking simulations utilizing the designed cMslA Gly12-Trp21 peptide because the preliminary part. The docking examine supplied a cMslA Gly12-Trp21 mannequin with peptides sure within the cleft fashioned by two monomers, in nearly the identical location and orientation as these of MslAΔTrp21 and MslA Trp21G within the MslH:MslAΔTrp21 and MslH:MslA Trp21G constructions, respectively. As anticipated, the indole ring of Trp21 was accommodated within the hydrophobic pocket (Fig. 5 and Supplementary Fig. 15). Within the mannequin construction, the side-chain of Phe20 within the cMslA Gly12-Trp21 molecule fashioned a parallel-displaced π-stacking interplay (2.7 Å) with the indole ring of its personal C-terminal Trp21. Particularly, the spine construction containing the carboxyl group of MslA Trp21 is predicted to bind at a location in line with that of MslA Gly21 within the MslH:MslA Trp21G advanced construction, and the carboxyl oxygen atom of the MslA Trp21 is positioned at a 3.0 Å distance from the unidentified steel ion (Fig. 5b and Supplementary Fig. 16).
Floor illustration of the binding cleft for MslA (a) and the proposed MslA Trp21 binding pocket (b) within the simulated MslH:cMslA Gly12-Trp21 construction (carbon-backbone of modeled MslH: mild purple; carbon-backbone of modeled MslA Gly12-Trp21: darkish purple) and its superimposed views with the MslH:MslAΔTrp21 crystal construction (carbon-backbone of MslH: cyan and pink; carbon-backbone of cMslA Gly12-Phe20: orange) and the MslH:MslA Trp21G crystal construction (carbon-backbone of MslH: inexperienced and yellow; carbon-backbone of cMslA Gly12-Gly21: blue inexperienced).
Subsequent, we investigated the metal-binding web site in MslH by evaluating the constructions of MslH and the structurally closest metal-dependent phosphodiesterase, YmdB26. This comparability indicated that the residues and water molecules concerned within the formations of the six-coordination motif and associated hydrogen-bond networks from the water molecules within the MslH:MslAΔTrp21 crystal construction are positioned at positions similar to these within the Fe(II) metal-binding-site consisting of Asp8, Glu39, Asn40, Asn67, His150, His175, His177, His68, Asp71, and a water molecule of YmdB (Supplementary Fig. 11). Particularly, the positions of Asn87 and His259 lining the six-coordination motif and His88 and Asp91 forming the hydrogen-bond networks from the water molecules in MslH are practically similar to these of Asn67 and His150, and His68 and Asp71 of YmdB, respectively (Supplementary Fig. 11d). Though the side-chain of Asp11 in MslH was barely rotated as in contrast with that of Asp8 in YmdB, each residues are positioned in nearly the identical place. The primary-chain of His261 in MslH, equivalent to His175 in YmdB, has a considerably totally different place from that of His175 in YmdB, as a result of substantial conformational change between the loop containing His261 in MslH and the corresponding loop containing His175 in YmdB. In the meantime, the side-chain of His261 can also be in nearly the identical place as that of His175 in YmdB, to take part within the six-coordination. In distinction, MslH lacked enough area to bind the second Fe(II) present in YmdB, presumably attributable to a number of conformational modifications comparable to the dearth of the amino acid residue on the location occupied by Asn40 in YmdB, the rotational and positional modifications of the side-chain of Glu44 equivalent to Glu39 in YmdB, and the rotation of the side-chain of His295 in MslH by 90 levels, as in contrast with that of His177 in YmdB. Thus, the MslH crystal construction recommended that MslH possesses a rearranged structural analogue of the Fe(II)-binding web site in YmdB. These rearrangements have been additionally conserved within the MslH:apo and MslH:MslA Trp21G constructions (Supplementary Figs. 12 and 13).
Ca(II)-binding of MslH
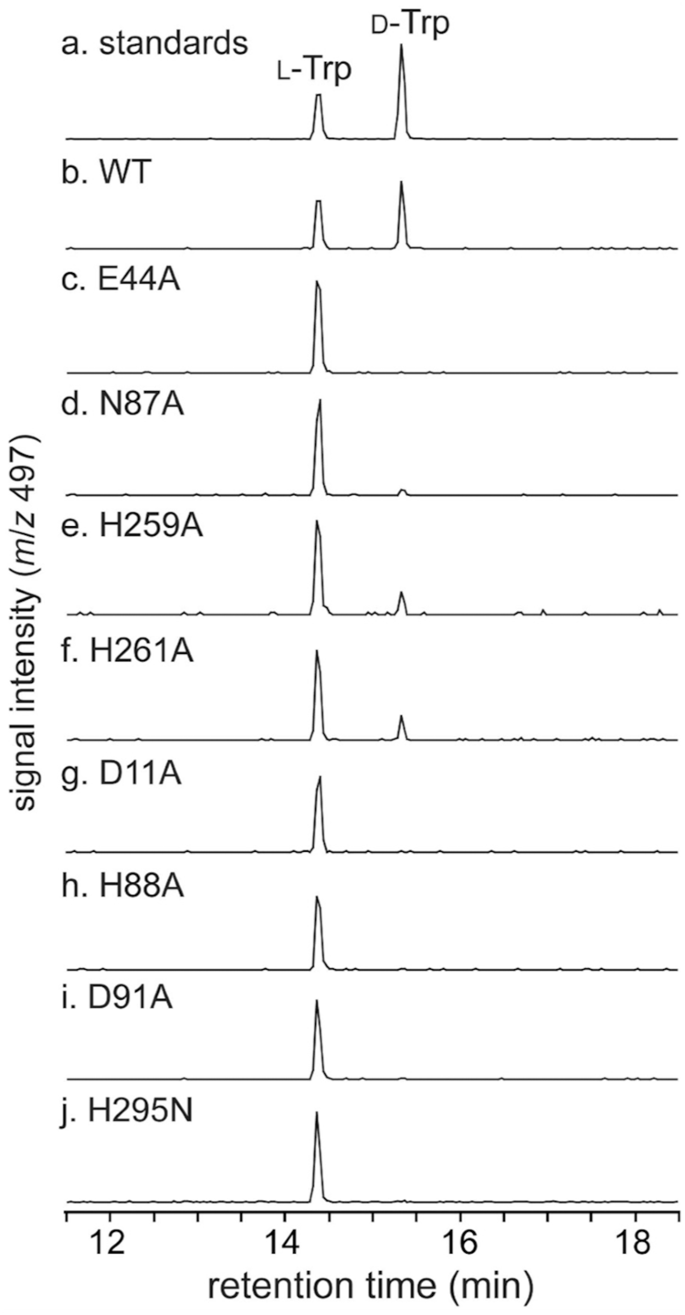
To additional verify the significance of the noticed six-coordinated motif through the enzyme response, we carried out mutagenesis research of MslH, during which 4 residues, Glu44, Asn87, His259, and His261 within the six-coordination motif have been every substituted with alanine. The mutagenesis research revealed that the MslH Glu44A and Asn87A variants lacked the epimerase exercise and the MslH His259A and His261A mutants diminished the exercise, suggesting the essential roles of the six-coordination motif for its catalysis (Fig. 6).
In the meantime, we carried out ICP-MS analyses of purified MslH to make clear the metal-dependency within the MslH catalytic mechanism. The ICP-MS measurements unveiled the presence of Ca(II) and Mn(II) ions at ratios of 0.4 mole and 0.3 mole per mole of MslH, respectively, suggesting both the Ca(II)- or Mn(II)-dependence of MslH (Supplementary Desk 3). Presumably, these steel ions have been integrated into MslH throughout enzyme expression in E. coli. Thus, to establish the steel ion liable for the MslH epimerase exercise, we expressed MslH in E. coli cultured in M9 medium containing solely NaCl, MgSO4, and CaCl2, that are important metals for E. coli progress. The in vitro enzymatic response indicated that the catalytic exercise was maintained in MslH expressed in M9 medium, as within the case of the recombinant MslH expressed in Luria-Bertani (LB) medium (Supplementary Fig. 17). As well as, to achieve details about the steel ions built-in into the crystal construction, the MslH:MslAΔTrp21 crystal construction refinements have been carried out by accommodating Ca(II), Mg(II), Mn(II), and Fe(II) ions within the aforementioned remaining electron density within the six-coordination motif. The Fo-Fc density maps displayed a optimistic worth for Mg(II) and adverse values for Mn(II) and Fe(II), however have been neither optimistic nor adverse for Ca(II) (Supplementary Fig. 14b–e). Because the B-factor values of metal-coordinating amino acid residues and water molecules are usually close to the steel’s B-factor worth, we additionally investigated their B-factor values. Consequently, the B-factor worth of the Ca(II) ion within the crystal construction was the closest to the typical of these of the metal-coordinating residues and water molecules, whereas that for Mg(II) was under and people for Mn(II) and Fe(II) have been above the typical (Supplementary Desk 4). Taken collectively, the aforementioned information allowed us to conclude that MslH is a Ca(II)-dependent epimerase.
Catalytic mechanism of MslH
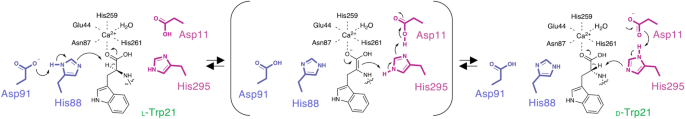
As beforehand reported, MslH catalyzed the reversible epimerization of the L- and D-amino acid orientations of MslA Trp21 in in vitro reactions19. To additional assist the reversible epimerization of MslH, we additionally carried out the in vitro MslH response utilizing MslA in D2O, to observe proton abstraction and addition on the Cα of the Trp21 residue. The next LC-MS evaluation revealed that MslH produced not solely MslA with C-terminal deuterated D-Trp, but in addition deuterated L-Trp (Supplementary Fig. 18). Thus, MslH catalyzes the reversible epimerization of the C-terminal L-Trp21 residue of MslA in a Ca(II)-dependent method. Subsequently, a configuration facilitating the epimerization is perhaps positioned close to the L-Trp21 of the substrate in MslH, individually from the six-coordination. The crystal constructions of MslH:MslA Trp21G and the docking mannequin of MslH:MslA Trp21 recommended that His88 coupled with Asp91, equivalent to the YmdB catalytic residues, His68 and Asp7126, was close to the α-proton of the L-Trp21 mannequin, whereas His295 coupled with Asp11, equivalent to the YmdB second Fe(II) ion-coordinating His177 and Asp8, was reverse from the α-proton, and these histidine residues have been positioned throughout the Cα facilities of the MslA Gly21 and MslA Trp21 residues in a linear method, respectively (Fig. 7). Related circumstances have been additionally present in different epimerases that catalyze reversible epimerization. For examples, the catalytic means of the PLP-independent epimerase, apMurI27, and a Mg(II)-dependent epimerase, AE epimerase28, allow reversible D- and L-epimerization of an amino acid by positioning the 2 catalytic cysteine residues and the 2 catalytic lysine residues in opposition to the response level of the substrate in a linear method, respectively. Subsequently, the noticed linear configuration of the histidine residues coupled with aspartate residues would enable MslH to facilitate the reversible epimerization of the L- and D-amino acid types of MslA Trp21.
The blue and magenta dashed traces characterize the gap between the Nε histidine atoms and the Cα middle of MslA Gly21 within the MslH:MslA Trp21G advanced construction (a) and the gap of the simulated MslA Trp21 within the cMslA Gly12-Trp21 docking mannequin superimposed with the MslH:MslA ΔTrp21 crystal construction (b) with the polar interplay between the Nδ histidine atoms and one of many Oδ aspartate atoms (distances in Å). Different targeted distances are additionally represented.
The 2 pairs of His88/Asp91 and His295/Asp11 residues noticed in MslH presumably act because the catalytic residues. Therefore, we performed further mutagenesis research on the 4 residues (MslH Asp11, His88, Asp91, and His295). Within the mutagenesis research, all residues apart from His295 have been substituted with alanine, whereas His295 was substituted with asparagine, with the identical dimension as histidine, due to the instability of the His295A variant through the purification and enzyme response. The in vitro experiment revealed that each one variants lacked epimerase exercise, indicating that the 4 residues are essential for catalysis (Fig. 6). Contemplating that His88/Asp91 in MslH correspond to the YmdB catalytic residues, His68 and Asp7126, the lack of the MslH exercise by the His88 and Asp91 level mutations explains the features of His88/Asp91 as catalytic residues. In distinction, the lack of catalytic exercise of the His295N and Asp11A variants may very well be attributable to a structural collapse of the metal-binding and substrate-binding websites, brought on by a breakdown of the hydrogen bonding community between six-coordination motif and Asp11/His295 by way of the w2 water molecule and substrate (Fig. 4a, b). Subsequently, we evaluated the His295N and Asp11A variants from a structural facet. We obtained their crystal constructions, MslH Asp11A:apo and MslH His295N:apo at 2.41 and a couple of.55 Å resolutions, respectively (Supplementary Desk 2). Each crystal constructions indicated that the variants retained the identical six-coordination motif as that within the wild-type MslH crystal construction (Supplementary Figs. 19 and 20). The His295N substitution had no impact on the energetic middle group, whereas the Asp11A substitution led to the pliability of His295 within the MslH Asp11A:apo crystal construction, the place a 1:1 various state was noticed for the His295 residue (Supplementary Fig. 19). The primary chain carbonyl group of the Pro294 residue within the MslH Asp11A:apo crystal construction was inverted by 180 levels, as in contrast with that of the wild sort (Supplementary Fig. 19e). Furthermore, the comparability of the MslH His295N:apo construction with the MslH:MslA Trp21G advanced construction confirmed that the gap between the Cα middle of MslA Gly21 and Oδ of Asn295 atom is 4.5 Å, which is longer than that (3.4 Å) within the MslH:MslA Trp21G construction (Fig. 7a and Supplementary Fig. 20f). These observations recommended that the Asp11 and His295 residues would play vital roles in sustaining the suitable catalytic state of MslH.
Based mostly on the excellent information described above and former studies, we suggest that MslH employs an “acid/base” chemistry just like that of UDP-galactose 4-epimerase29, within the epimerization response to generate epi-MslA, as follows (Fig. 8): MslH accommodates the Gly12-Trp21 portion of the MslA/MslB1 advanced substrate throughout the cleft, and initiates the α-proton abstraction of L-Trp21 within the MslA/MslB1 advanced by Asp91-activated His88, to provide an enolate intermediate in a transition state with the Ca(II) ion. Subsequently, a proton switch from the His295 side-chain, positioned on the other facet, happens on the α-position of the enolate intermediate to provide epi-MslA by way of the tautomerization of the enolate type to the keto type of the intermediate, and the His295 side-chain additional abstracts a proton from the Asp11 side-chain. Lastly, MslH releases epi-MslA as the tip product, which is subsequently subjected to the lasso cyclization steps, and recovers the preliminary state of His88-Asp91 by proton switch ranging from His295 to Asp91, by way of the Asp11-w2-Ca(II)-w1-His88 community noticed within the crystal construction. In distinction, when MslH accepts epi-MslA because the substrate, the enzyme facilitates the epimerization response of epi-MslA to MslA, by using Asp11-activated His295 as the bottom catalyst (Fig. 8). As a facet notice, though the preliminary proton abstraction might happen with His295, His88 probably acts because the preliminary catalytic base, primarily based on our docking examine (Fig. 7b).
[ad_2]