[ad_1]
Batch experiments/operational circumstances
The right operational circumstances have been studied by the batch experiments; these circumstances are the preliminary pH, sorbent dose, the impact of contact time, agitation velocity and the preliminary focus of the TC (C0). In all experiments, 250 mL flasks had been used, and 50 mL of contaminated water with various TC concentrations (10, 50, 100, 150, and 200 mg/L) had been examined; 0.1 g of the synthesised (CFO-SS) was utilized with an agitation velocity of 200 rpm. After the three h, the water was filtered utilizing grade (5) filter paper to separate any impurities. Ultraviolet–seen spectrophotometer (UV) kind (HACH DR3900) has been used to detect the ultimate focus of TC (Ce). The quantity of TC adsorbed by the (CFO-SS) reactive media has been calculated utilizing Eq. (9), whereas the removing effectivity has been calculated utilizing Eq. (10)35,36:
$${q}_{e}=({C}_{0}-{C}_{e})frac{V}{m}$$
(9)
$$R({%})=frac{({C}_{0}-{C}_{e})}{{C}_{0}} occasions 100$$
(10)
The place’s, ({(q}_{e})) is the quantity of adsorbate loaded on the adsorbent (mg/g), (({C}_{0}, {C}_{e})) are the preliminary and remaining focus of the adsorbate (mg/L), (V) is the amount of aqueous answer (L), and (m) is the mass of adsorbate (g). the (%) removing effectivity of the contaminant is ((R)).
The time required to attain the equilibrium state for (10, 50, 100, 150 and 200 mg/L) concentrations has been investigated. All experiments have been carried out in pH (7), (CFO-SS) dose of 0.1 g for every 50 mL of contaminated water with TC, agitation velocity (200 rpm). Outcomes confirmed that the adsorption charge was quick within the first 60 min after which slowed down as a result of discount within the accessible websites on the adsorbate and the occupation of many of the websites by TC molecules. Outcomes confirmed that 180 min had been enough to succeed in the utmost TC loading on the (CFO-SS) particles. Whereas rising the focus of TC in answer, the removing effectivity was decreased from 90% when the focus of TC was 10 mg/L to 21% when the focus was 200 mg/L, which pertains to the truth that many of the accessible websites on the (CFO-SS) are being occupied and there are not any accessible websites for extra TC molecules. One other essential issue within the batch experiments has been investigated, the pH worth of the aqueous answer, a variety of aqueous pH (2–12) have been investigated, TC preliminary focus (C0) was 50 mg/L, and (CFO-SS) dose was 0.1 g, at 200 rpm agitation velocity. Outcomes confirmed that rising the pH worth of the aqueous answer enhanced the removing effectivity; the utmost removing was at pH (10), and after that, the removing effectivity reached an equilibrium state. To justify this behaviour. A Zeta Potential take a look at has been carried out utilizing the strong addition technique (ΔpH) by a (0.15) g of (CFO-SS), 0.01 M NaCl, agitation velocity at 200 rpm for twenty-four h. Outcomes revealed that the cost of the (CFO-SS) is destructive under 5 and constructive above it. TC is a broad-spectrum antibiotic which consists of 59.45% of (C), 5.44% (H), 6.30% (N) and 28.80% of (O), with amphoteric, phenolic, and alcoholic properties37. Owing to the presence of a dimethylammonium group, a phenolic diketone moiety, and a tricarbonyl system, TC exists largely as cationic (({mathrm{TCH}}_{3}^{+})) at pH lower than 3.3, zwitterionic (({mathrm{TCH}}_{2}^{pm }))) between pH 3.3–7.7, and anionic (({mathrm{TCH}}^{-})) and (({mathrm{TCH}}_{2}^{-})) at a pH larger than 7.738,39. The upper electrostatic attraction between TC and the (CFO-SS) is as a result of excessive depth of the (+) cost on the adsorbate, which can appeal to with the (-) cost of the (({mathrm{TCH}}_{2}^{-})). The impact of sorbent dose and the optimum dose that take away many of the pollutant in a 50 mL of polluted water having 50 mg/L TC, pH 7, agitation velocity of 200 rpm for 180 min. The sorbent dose has elevated from 0.05 to 0.5 mg. Outcomes confirmed {that a} dose of 0.3 g (CFO-SS) can take away 91.8% of the TC, whereas the removing effectivity was 53.4% with a dose of 0.05 g. After 0.3 g, an equilibrium has been reached as a result of many of the TC molecules have been adsorbed, and an equilibrium is achieved between the sorbent and the aqueous atmosphere. The velocity of agitation is a crucial issue40 that impacts the adsorption of TC on the engineered (CFO-SS), at pH (7), 0.3 g of sorbent for 50 mL containing 50 mg/L TC, the agitation velocity has been altered from (0) to (250) rpm, outcomes reviled that the optimum agitation happens at (200)rpm. That is as a result of excessive agitation that brought about disruption to the pollutant; plainly the agitation at 200 rpm offered the right contact time between the pollutant and the adsorbate, accordingly. The removing effectivity at 200 rpm was 91.8%, whereas it was 81.1% and 90.7% when the agitation was 150 rpm and 250 rpm, respectively. The batch experiments have been extensively detailed within the writer’s earlier article41
Sorption isotherm
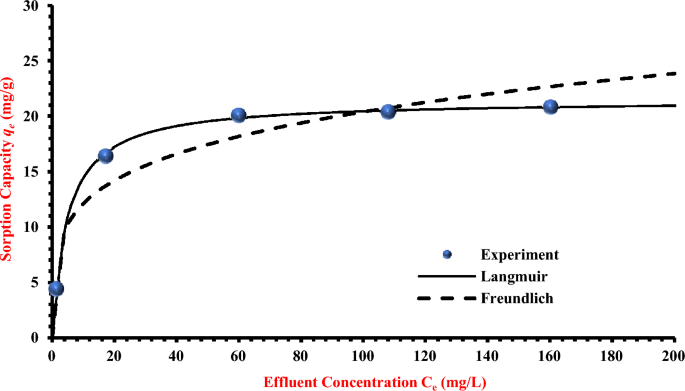
The adsorption isotherm is used to signify the distribution of contaminants on the strong part at equilibrium and to compute the maximal adsorption capability and the adsorbent’s affinity. These isotherms are crucial relationships to characterise the interplay within the packed mattress between the contaminants and the coated sand. The acquired sorption outcomes of TC adsorption by the synthesised coated sand are fitted utilizing nonlinear variations of Freundlich and Langmuir fashions by using the “solver” choice nonlinear regression in Microsoft Excel Software program 365. Desk 1; exhibits the mannequin constants, the sum of squared errors (SSE), and R2. Outcomes recommend that the Langmuir mannequin can higher signify the obtained measurements than the Freundlich interpretation. In line with the values of R2 and SSE (0.995 and 0.202), respectively), the Langmuir mannequin seems to be extra fitted to measurement formulation than the Freundlich mannequin. The matching between the measurements and the Langmuir mannequin, then again, is described in Fig. 3; the best capability and affinity fixed values for the interplay of TC with current sorbent had been 21.450 mg/g and 0.204 L/mg, respectively, which could be very near the utmost experiments (qe) (21.96 mg/g).
Characterisation of sand coated with calcium ferric oxides
X-ray fluorescence evaluation and XRF evaluation have been carried out to research the chemical composition of the used WPSA; outcomes confirmed that the predominant oxide forming the WPSA is the CaO which represents about 34% of the entire composition, as proven in Desk 2; under.
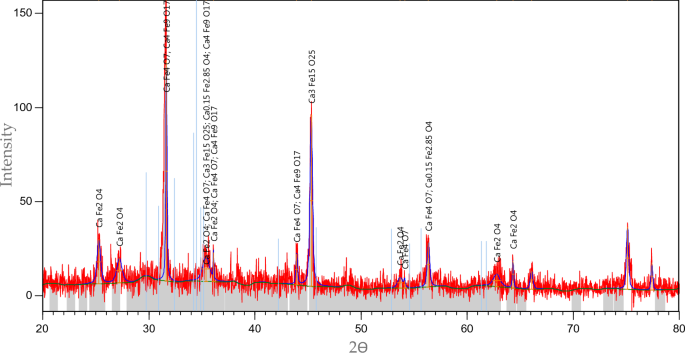
The XRD spectral take a look at for the (CFO-SS) has been carried out, as illustrated in Fig. 4. Outcomes have been analysed utilizing the PANalytical/X’Pert HighScore Plus software program for XRD powder diffraction measurements; it’s revealed that calcium ferric oxides have appeared on the sand after the modification course of. Measurements have been in contrast with the (Joint Committee on Powder Diffraction Requirements (JCPDSs)) and located that the silica oxide is the key ingredient accountable for the presence of peaks. The X’Pert HighScore Plus software program revealed the synthesis of (CaFe4O7, Ca3Fe15O25, Ca0.15Fe2.85O4, CaFe2O4 and Ca4Fe9O17). These compounds are accountable for the adsorption of TC because it turned the uncooked sand right into a reactive media have the power to catch the pollution in an aqueous atmosphere as a result of intensification of Fe ion42 and Ca ion43.
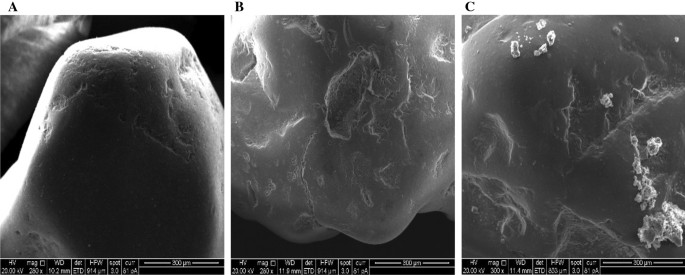
The morphology of uncooked, synthesised sand earlier than and after its mixture with TC has been investigated utilizing FEI Examine-S (SEM) variable vacuum (0.1–30 kV vary); leads to Fig. 5 revealed a easy floor of the sand, the coated sand elevated floor roughness as a result of immobilising of calcium ferric oxides on the sand floor, this led to the attraction of TC to the coated sand.
Infrared spectroscopy investigates the interplay of infrared radiation with sand, coated sand, and coated sand after TC adsorption. Outcomes confirmed that the IR adsorption for these analysis samples follows the everyday IR adsorption as proven in Fig. 6; in different phrases, the presence of Si–O vibration bending occurring at 777 cm−1, 779 cm−1 and 693 cm−1 reveals the presence of quartz sand19,29,44. The stretching vibrations of (OH) teams could cause high-intensity absorption bonds29. The height of sand coated with calcium ferric oxides at 3375 cm−1 is as a result of stretching mode of the OH group, stretching vibration of a hydrogen bond or formation of interlayer water molecules. The peaks at 1162 cm−1 are attributed to the C–O tensile vibrations and C–O rings ensuing from the deformation of C–C–H and C–O–H45. Uneven and symmetric stretching vibrations of aliphatic C–H had been noticed at 2957 cm−145. The Si–O–Si bond is seen on distinct 1086, 797, 695, 1069, 799, 1082, and 797 absorption bands. Silanol teams (Si–OH) which seem at wavenumber 846 cm−1, could possibly be activated on the floor of the grains of the matrix46, which may trigger the attachment of TC chains to the floor of grains of sand with the formation of hydrogen bonds of the sort Si–O–H⋅⋅⋅O–H. Moreover, the presence of a (C–O–Si) bond at a wavenumber of 1164 cm−1 may end up from binding interactions between the coating matrix and the matrix (silane Si–OH teams). Primarily based on the FTIR evaluation, it’s indicated that presence of a large spectrum of practical teams. This evaluation concluded that every one media producing totally different band spectra would give totally different adsorption intensities attributable to totally different floor practical teams.
Affect of mounted mattress operation circumstances
The aim of the column assessments was to simulate the transport of contaminants in a one-dimensional move, which intently resembles the precise functioning of a permeable reactive barrier. The contaminated water was pumped utilizing a WATSON MARLOW peristaltic pump mannequin 520S with move charges of 1.58 mL/min, 4.75 mL/min, and 9.5 mL/min. These move charges had been the minimal that could possibly be achieved by the pump whereas sustaining a creeping/laminar move with Reynold’s variety of lower than 10. The efficiency of the reactive media was evaluated by monitoring the effluent’s normalised contaminant focus over time till the reactive media was depleted. The outcomes of those assessments present worthwhile perception into the efficacy of permeable reactive limitations within the remedy of contaminated water.
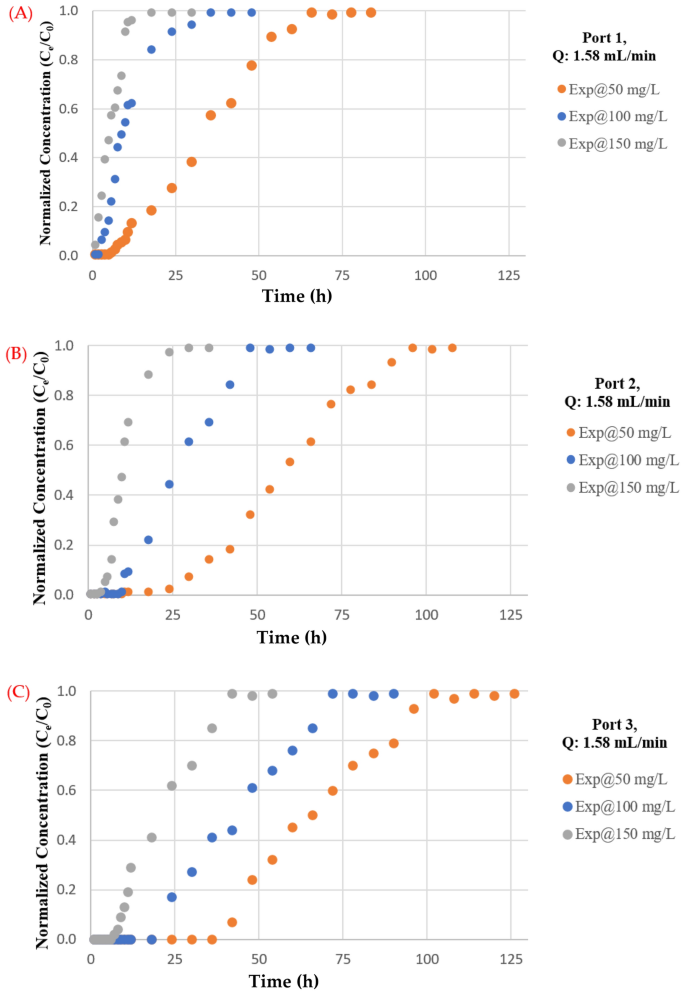
Affect of influent flowrate
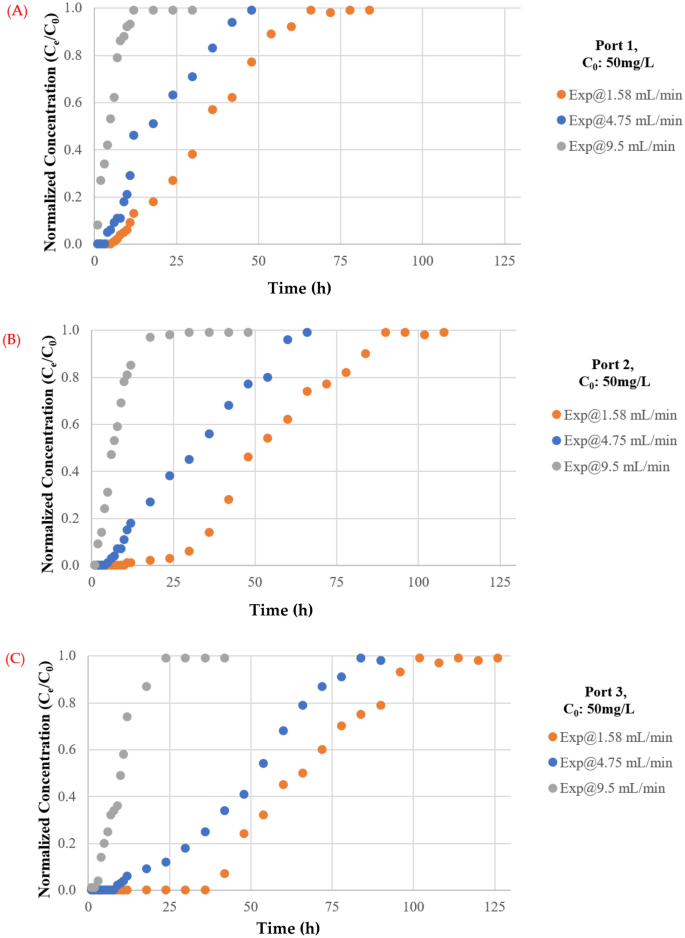
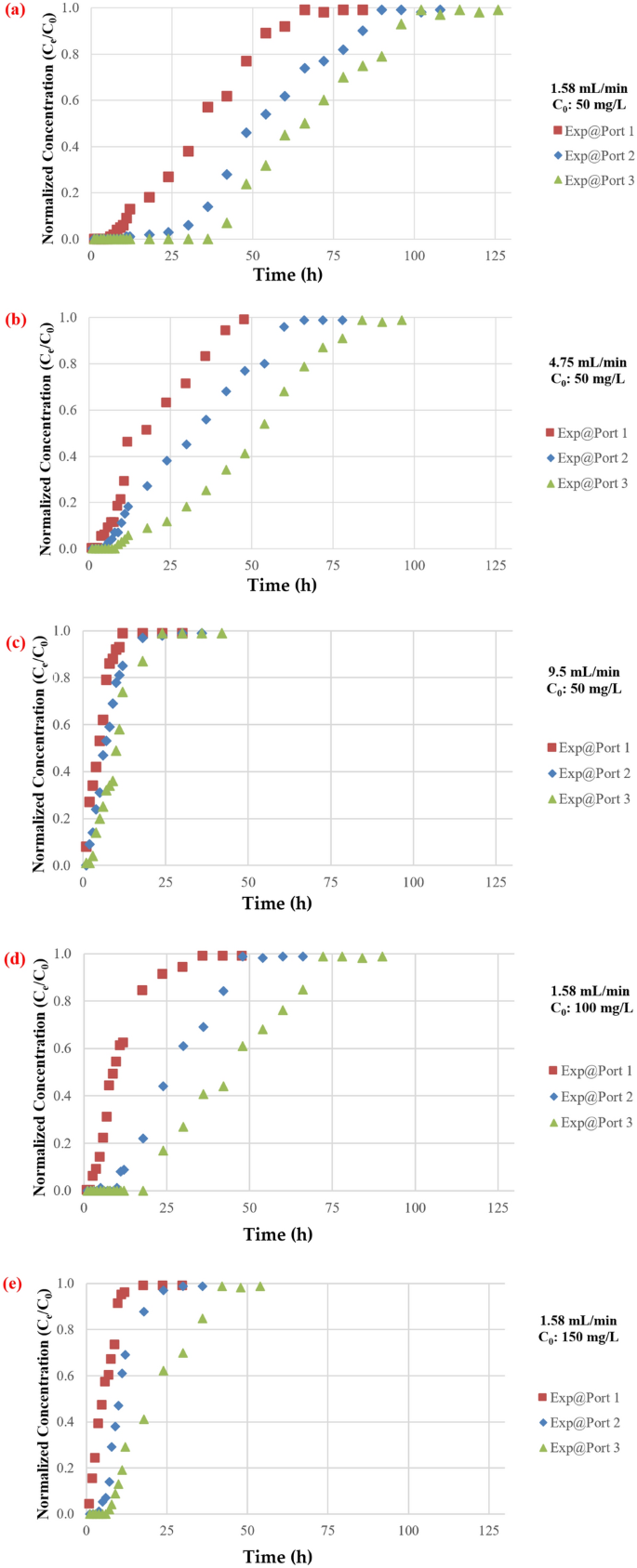
The impact of flowrate on the mass of TC maintained within the barrier has been investigated utilizing flowrates of 1.57, 4.75, and 9.5 mL/min. The plotting of breakthrough curves based mostly on measurements of normalised concentrations with various move charges at ports 1, 2, and three are proven in Fig. 7. It’s apparent {that a} excessive discharge can shorten the time required for the breakthrough and enhance the steepness of the curve as a result of contaminated water leaves earlier than reaching equilibrium, as predicted. This phenomenon is brought on by inadequate antibiotic residence time within the column at a excessive flowrate47, limiting antibiotic molecule diffusion into the pores of the synthesized coated sand. Moreover, the adsorbed TC on the adsorbent could also be desorbed to the aquatic atmosphere as an impact of the excessive flowrates which can cut back the removing effectivity48. Nevertheless, the upper flowrate had a shorter mass switch zone. The bottom flowrate had the best adsorption of complete antibiotic. As water velocity elevated, adhesion between solute and sorbent might lower, leading to a noticeable lower in sorption effectiveness. As well as, desorption of sure molecules of a sorbet contaminant is feasible, significantly for unfastened and reversible bonds with the sorbent. Because of this, the focus of TC within the effluent would possibly quickly improve, leading to an early breakthrough time. The low flowrate aided pollutant adsorption within the mounted mattress, which is in line with prior analysis akin to49,50,51,52.
Affect of pollutant focus
The impact of feed antibiotic preliminary focus depicted in Fig. 8. Because the influent antibiotic focus elevated from 50 to 150 mg/L in port (3), the exhaust time for TC decreased from (102) to (36) h. A larger driving drive for antibiotic mass switch resulted from a better influent focus31,53. Quite the opposite, when the focus of the influent antibiotic decreased, the breakthrough curves rise later, indicating a wider switch zone and gradual intraparticle diffusion course of54. It must be famous that the adsorption functionality will increase with the focus of influent antibiotics. It is because a bigger focus gradient resulted in quicker mass switch attributable to an elevated floor diffusion coefficient. Furthermore, when the focus of influent antibiotics will increase, so does the general quantity of antibiotics adsorbed. At move charges of 1.58, 4.75, and 9.5 mL/min for ports 1, 2, and three, the impact of influent focus on the entrance of the breakthrough curves is examined. As a consequence of a slower adsorption, the curve is much less noticeable at decrease entry concentrations; nonetheless, because the focus rises, the steepness of the curve will increase, and the mattress acquires saturated extra shortly. Moreover, a notable fall within the magnitude of the mass switch coefficient would possibly happen along with a lower within the focus gradient. The transmission of the contaminant entrance might be delayed because of this for this discount, and the “exhaustion time” will shorten because the influx focus rises, leading to a discount within the quantities of contaminant which might be adsorbed contained in the mattress. The outcomes from the influent focus impact have been confirmed by evaluating it with the outcomes from literature akin to55,56.
Affect of the mattress depth
Rising the mattress depth from 15 to 30, and 45 cm resulted in a considerable lower in TC focus in the barrier for all flowrates and baseline focus values used on this analysis as proven in Fig. 9. This is likely to be as a result of contaminated fluid remaining within the mattress for an prolonged size of time, which can improve the absorption course of as a result of improve of the accessible floor space of the adsorbent and improve within the contact time57. Nevertheless, barrier’s performance diminished with time as a result of saturation of the mattress with sorbet pollutant, this discovering have been confirmed by58,59.
Behaviour similarity with conventical mounted mattress column fashions
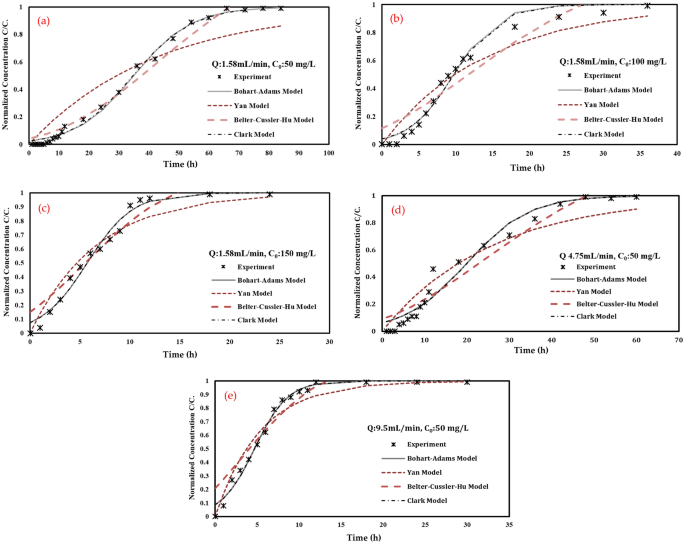
4 conventional adsorption fashions had been used for simulating adsorbate transport in a set mattress column and employed to suit the adsorption experiments knowledge as a comparability with an anticipated mannequin; these fashions are Bohart–Adams Mannequin, Yan Mannequin, Belter–Cussler–Hu Mannequin and Clark Mannequin.
As proven in Desk 3 and Fig. 10, in all ports, experimental knowledge appears to finest match with the Adams–Bohart mannequin and Clark mannequin because it gave the biggest sq. root quantity (R2) (for port 1: R2 had been 0.9953, 0.9652, 0.9755 and 0.9945 for Adams–Bohart, Yan, Betler-Cussler-Hu and Clark fashions, respectively). This assumes that the equilibrium shouldn’t be instantaneous and the adsorption charge is proportional to the focus of adsorbate.
These fashions are extensively used to explain the breakthrough curve. Adams–Bohart’s mannequin nicely described the transport of TC within the column. It is usually noticeable that the values of (kC0) improve with rising the TC influent focus and discharge charge and, usually, lower with the rise of mattress depth. The outcomes additionally indicated that the adsorption of TC, within the column, decreases with the rise of the influent focus of TC, the move charge and the lower of the mattress.
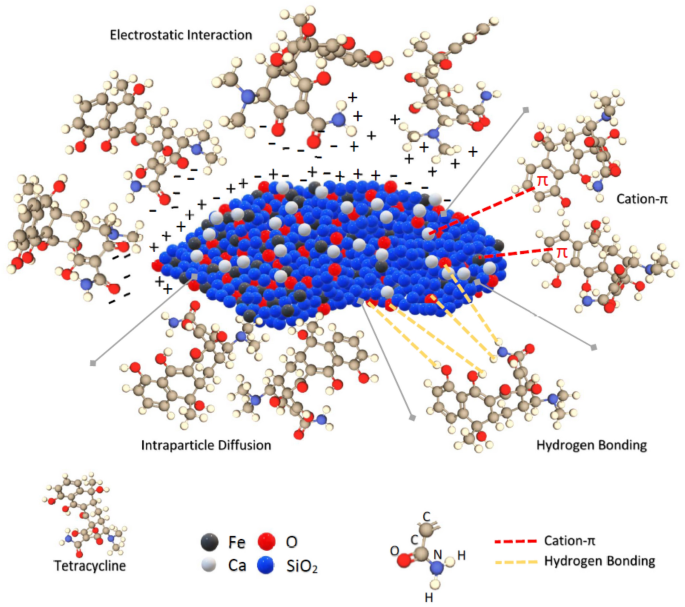
Potential mechanism of adsorption
The adsorption of TC on the synthesised (CFO-SS) layer is influenced by 4 interactions; these are the electrostatic interplay, cation-π interplay, H-bonding and intraparticle diffusion. The characterisation assessments carried out on the uncooked sand, CFO-SS, earlier than and after the adsorption offers an apparent clue to the adsorption mechanism; at first, the XRD take a look at for the uncooked sand and for the coated sand proves the synthesise of calcium ferric oxides on the sand floor, the enrichment of sand floor by compounds wealthy with calcium and ferric ions along with the oxygen are accountable for the catchment of TC from the aqueous atmosphere. TC has (3) pKa values at (3.3, 7.7 and 9.7), which signifies that TC can chemically work together at totally different pH values by donating its protons. The Zeta potential take a look at proves that the floor cost of the (CFO-SS) is destructive below (5) and constructive over (5). It will allow the CFO-SS to work together with (H +) and (O−) ions on the TC, which ends up in an electrostatic attraction between the TC and the (CFO-SS) floor. The SEM proved the change in floor morphology of the coated sand as a result of formation of calcium ferric oxides and the drying of the engineered sand, which led to a rise the floor roughness. This led the molecules of TC to interlock inside the coated unhappy grooves by the intraparticle diffusion; the intraparticle diffusion kinetics proves this phenomenon. This analysis examines the distinction within the FTIR spectra of CFO-SS earlier than and after the adsorption of TC. The obtained spectra, together with the FTIR spectrum of TC, are offered in Fig. 11. Nevertheless, the iron and calcium components within the Fe-OH and Ca-OH seemingly offered an uninhabited orbital for lone pair of electrons and π-bonding electrons in TC practical teams (hydroxyl, carbonyl, and amino teams) and benzene ring forming TC-Fe, TC-Ca complexes to strengthen the cation-π interplay. This rationalization fully agrees with the truth that the iron and calcium components demonstrated that the antibiotic-metal complicated may facilitate the elimination of antibiotics60,61.
[ad_2]