
[ad_1]
The small protein ubiquitin is especially well-known for marking proteins for degradation however it has additionally been proven to manage just about all mobile processes. In parallel to the ubiquitin system varied different ubiquitin-like modifiers have developed, of which Fubi is especially poorly studied regardless of its immunomodulatory exercise.
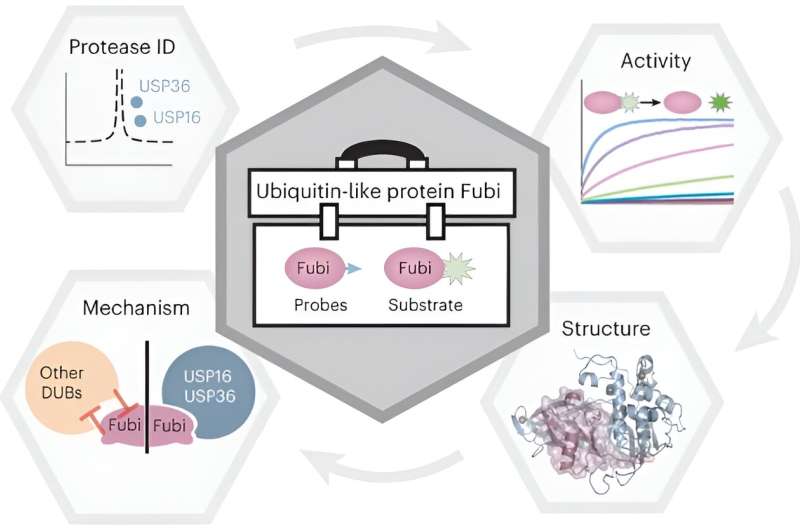
Scientists round Malte Gersch, analysis group chief on the Chemical Genomics Centre on the Max Planck Institute of Molecular Physiology, have now gained first molecular insights into the equipment facilitating the Fubi-controlled maturation of a key protein of the ribosome, the cell’s protein manufacturing unit. With the assistance of a newly developed chemical device equipment, the researchers characterised how two deubiquitinating enzymes present particular Fubi hydrolase exercise and thereby moonlight as Fubi proteases in a two-tier method.
Fubi is produced by cells as a fusion protein with the ribosomal protein S30, and should be separated from S30 by proteases for functioning ribosomes. In immune cells, this by-product of ribosome manufacturing is utilized as a secreted signaling molecule, for instance to regionally scale back the exercise of the maternal immune system within the uterus and to thus allow embryos to implant. How Fubi is particularly acknowledged by proteases and the way they distinguish it from ubiquitin was beforehand unknown.
Rachel O’Dea and Malte Gersch clarify their analysis intimately:
What’s the discovery that you just made and why is it thrilling?
Our group revealed how two deubiquitinating enzymes can even act as proteases of the ubiquitin-like protein Fubi and gained molecular insights into how that is doable in particular method. That is noteworthy as a result of, regardless of similarity between Ubiquitin and Ubiquitin-like proteins, the enzymes regulating them in people are normally not the identical.
We present that this twin exercise is particular to the 2 enzymes USP16 and USP36 and our crystallography research mechanistically clarify how this uncommon cross-reactivity is achieved. Surprisingly, in contrast to what’s noticed in cross-reactive enzymes from micro organism or viruses, we didn’t discover any extra structural parts that facilitate the extra Fubi exercise of those effectively characterised Ubiquitin proteases.
As a substitute, Fubi recognition is mediated by way of a small cryptic motif on a complementary binding floor.
What’s so particular about your developed device equipment?
Probably as a consequence of its difficult amino acid composition Fubi protein instruments had not but been added to the repertoire of instruments for learning Ubiquitin and Ubiquitin-like proteins.
Our work demonstrates facile approaches for making Fubi instruments which will be readily tailored by different scientists within the subject. The probes and Fubi fluorescent substrate described right here present a method for assessing Fubi eraser exercise in each mobile and in-vitro settings.
Why is your analysis necessary for the scientific group?
Our work supplies new molecular insights into how enzymes can have actions spanning a number of modification techniques. Explaining how USP16 and USP36 play a task in ribosomal protein maturation expands our understanding of mechanisms regulating this vital mobile course of.
Fubi has primarily been studied by scientists from the immunology subject, and extra lately of the ribosome subject, and our work approaching the subject with the Ubiquitin background enhances these different works. Collectively all knowledge converges right into a two-tier mannequin for Fubi processing.
Why is your analysis necessary for society?
Owed to their speedy and reversible nature posttranslational modifications similar to Ubiquitin and Ubiquitin-like proteins are vital regulators of just about all mobile processes.
Fubi has been linked to immunomodulatory capabilities and has been proven to switch proteins throughout immune stimulation responses. Understanding the precise function of Fubi on this course of will broaden our understanding of the how cells reply to immune signaling.
What are the subsequent steps you’ll take?
Our insights into Fubi recognition enable for tuneable Fubi protease exercise in cells and are thus paving the best way for higher understanding the mobile function of this enigmatic protein as a post-translational modification.
As well as, we’re utilizing the probes to facilitate investigation into the molecular mechanism by which different proteins work together with Fubi. However first we are going to have a good time.
The findings are printed within the journal Nature Chemical Biology.
Extra info:
Rachel O’Dea et al, Molecular foundation for ubiquitin/Fubi cross-reactivity in USP16 and USP36, Nature Chemical Biology (2023). DOI: 10.1038/s41589-023-01388-1
Offered by
Max Planck Society
Quotation:
Getting protein factories to run—How deubiquitinating enzymes moonlight as Fubi proteases (2023, August 11)
retrieved 12 August 2023
from https://phys.org/information/2023-08-protein-factories-runhow-deubiquitinating-enzymes.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.
[ad_2]