[ad_1]
Synthesis and construction characterization of UiO-66-X
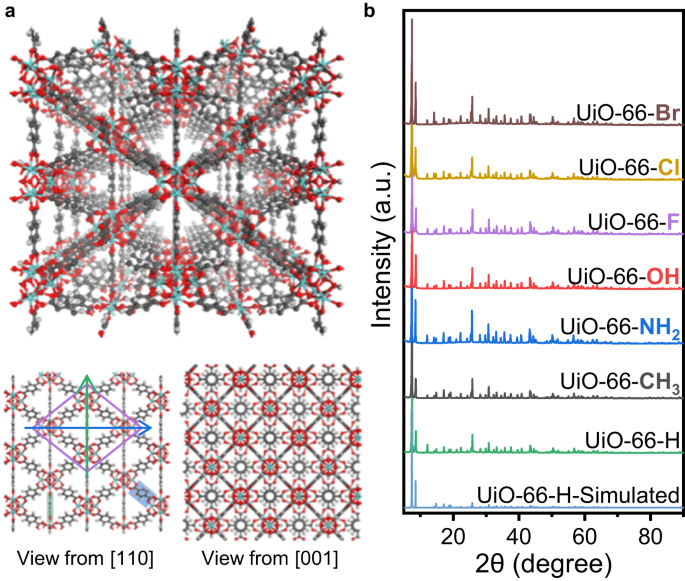
UiO-66 are fashioned by Zr nodes and BDC linkers, with tetrahedron and octahedron pores (Fig. 1a). Seen from the course of [110], UiO-66 will likely be projected right into a rhombus tessellated sample. The 4 vertices of a cell are all Zr nodes, and the 4 edges are BDC linkers with a level of 30°. The brief diagonal is one other column of BDC linkers, with their benzene rings parallel to the [110] projections. The brief diagonal passing by way of two carboxyl C and parallel to the (110) side is denoted because the brief axis (inexperienced arrow in Fig. 1a), whereas the lengthy diagonal perpendicular to the benzene rings of the BDC linkers is denoted because the lengthy axis (blue arrow in Fig. 1a). If the benzene rings of BDC linkers don’t rotate across the C2 axis (Supplementary Fig. 1), the depth profile alongside the lengthy axis will include one slim peak within the center, which corresponds to the benzene rings degenerating right into a line alongside the brief axis. Nonetheless, if the benzene ring flips with a level, the slim line will likely be blurred and broadened to a bigger vibrant spot.
a Schematic mannequin of UiO-66. The views from [110] and [001] projections are proven under. The lengthy and brief axes from the [110] projection are marked by blue and inexperienced arrows, respectively. The gray, crimson and white atoms signify C, O and H parts, respectively. b XRD patterns of UiO-66-X and simulated outcomes of UiO-66-H. Supply knowledge are supplied as a Supply Information file.
When altering the linkers to different BDC-X linkers with completely different useful teams, we will acquire UiO-66-X samples, which have comparable construction to conventional UiO-66. To be constant, we denote UiO-66 with none useful group as UiO-66-H, in order the BDC-H linkers. By scanning electron microscopy (SEM), we discovered that the addition of useful teams hardly ever modifications the morphology that every one UiO-66-X samples seem as common octahedrons (Supplementary Fig. 2). X-ray powder diffraction (XRD) outcomes additionally affirm that the UiO-66-X samples have the identical crystal construction with fm-3m symmetry (Fig. 1b), indicating that the useful teams of BDC-X linkers hardly ever affect the crystal construction. Excessive angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) photographs present easy diamond-like construction from [110] projection (Supplementary Fig. 3), that are typical photographs of UiO-66 samples. By these characterizations, we affirm that UiO-66-X samples are synthesized with comparable construction, and the one distinction is the useful teams.
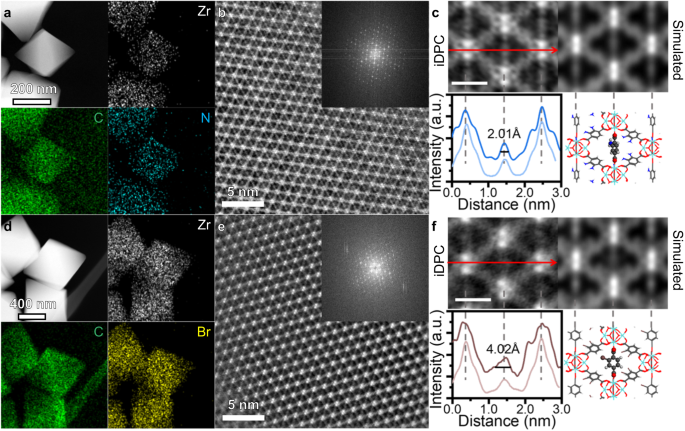
The power dispersive X-ray spectroscopy (EDS) is utilized to substantiate the existence of useful teams. The UiO-66-H samples present solely Zr, C and O indicators (Supplementary Fig. 4), whereas N, Cl, Br indicators are clearly detected in UiO-66-NH2, UiO-66-Cl and UiO-66-Br, respectively (Figs. 2a, d and Supplementary Figs. 5, 6). The composition of newly launched parts can also be in step with the theoretical mannequin, the place all linkers had been changed by functionalized BDC-X linkers (Supplementary Desk 1).
HAADF-STEM photographs and corresponding EDS mapping of UiO-66-NH2 (a) and UiO-66-Br (d). iDPC-STEM photographs of UiO-66-NH2 (b) and UiO-66-Br (e) from [110] orientation. The FFT sample is proven as inset. Magnified iDPC-STEM photographs and simulated outcomes of UiO-66-NH2 (c) and UiO-66-Br (f). The depth profile alongside the lengthy axis and the mannequin of BDC-NH2 rotation are proven under. The darkish strains within the depth profile are experimental outcomes, whereas the sunshine strains are the simulated outcomes. The dimensions bar is 1 nm. The gray, crimson, white, blue and brown atoms signify C, O, H, N and Br parts, respectively. Supply knowledge are supplied as a Supply Information file.
The direct imaging of rotation properties of BDC-X linkers
Contemplating that HAADF-STEM photographs can hardly replicate the construction of natural linkers in UiO-66-X as a result of e-beam harm, we utilized the iDPC-STEM know-how to picture the BDC-X linkers for higher understanding of the microscopic affect of functionalization. Taking UiO-66-NH2 and UiO-66-Br as examples, we will simultaneous picture the Zr nodes and BDC-X linkers from the [110] projection (Fig. 2b, e), the place FFT patterns affirm the excessive decision. The iDPC-STEM photographs are in step with the schematic mannequin that diamond-like cells are noticed. For the UiO-66-NH2 samples, the BDC-NH2 linkers alongside the brief axis are recognizable as 4 separate vibrant spots (Fig. 2c). These spots might be recognized as two carboxyl teams and the higher and decrease components of benzene ring. The depth profile alongside the lengthy axis exhibits a peak with a full width at half most (FWHM) of two.01 Å. The width of this peak represents the ‘width’ of the projection of BDC-NH2 linkers within the (110) airplane, which might be additional correlated to the dynamic properties of UiO-66-X. For UiO-66-Br, nevertheless, the indicators of BDC-Br are blurred from a slim line to a bigger vibrant spot (Fig. 2f). The uneven peak form signifies that the useful teams of -Br could also be biased in a single course. Furthermore, the FWHM alongside the lengthy axis elevated to 4.02 Å. The broadening impact is attributed to the π-flipping of benzene rings within the BDC-Br linkers. Opposite to BDC-NH2, the benzene rings of the BDC-Br linkers are almost parallel to the (110) airplane, resulting in a big vibrant spot in the midst of each lengthy and brief axes. To the very best of our data that is the primary instance of a way to straight see the uniform and disordered orientations of benzene rings in UiO-66-NH2 and UiO-66-Br, respectively, and the dramatic impact of functionalization on the dynamic properties is sudden.
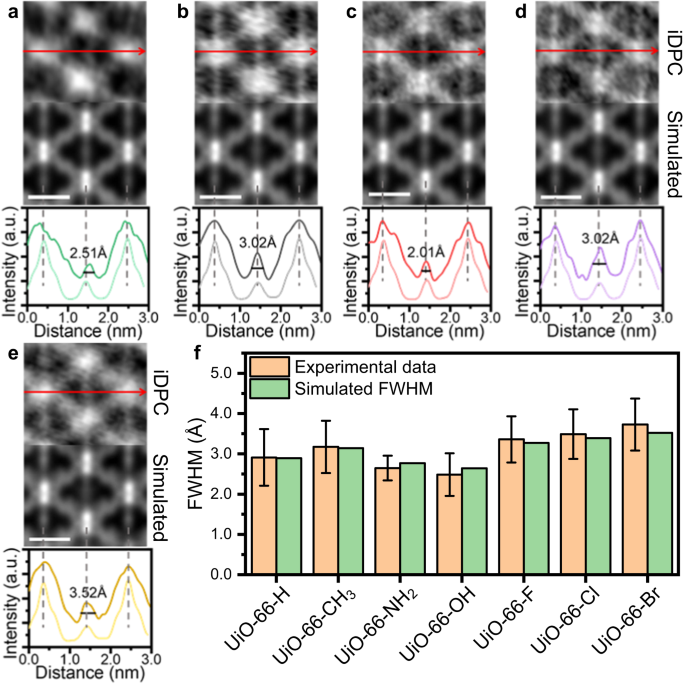
Along with UiO-66-NH2 and UiO-66-Br, we additionally use the iDPC-STEM know-how to characterize the UiO-66-X (X = H, CH3, OH, F, Cl) samples (Supplementary Fig. 7). The height on the middle of lengthy axes exhibits various levels of broadening with completely different useful teams on the BDC-X linkers, after which signifies completely different dynamic properties of UiO-66-X (Fig. 3a–e). This broadening is ascribed to the completely different rotation properties of benzene rings, and the values of FWHM can be utilized as a quantitative description of the diploma of benzene rotation. We summarized the depth profiles of greater than 20 areas, and calculated the common FWHM for various UiO-66-X samples (Fig. 3f, Supplementary Fig. 8). The experimental worth is in step with the simulated worth, the place a three-layered mannequin is utilized for simulation utilizing the ToTEM software program45 (Supplementary Fig. 9). The rotation levels of benzene ring for simulation are decided utilizing 0.18 eV as the common rotation power at RT, which will likely be mentioned later. By iDPC-STEM imaging, we will straight ‘see’ the completely different π-flipping properties of UiO-66-X. The rigidity of BDC-X linkers in UiO-66-X follows the development of -OH > -NH2 > -H > -CH3 ~ -F > -Cl > -Br. This development agrees properly with the beforehand reported outcomes of UiO-66-(OH)2 > UiO-66-H > UiO-66-(CH3)2 obtained from 2H-NMR30.
iDPC-STEM photographs, simulated outcomes and depth profile alongside the lengthy axis of UiO-66-H (a), UiO-66-CH3 (b), UiO-66-OH (c), UiO-66-F (d) and UiO-66-Cl (e). The darkish strains within the depth profile are experimental outcomes, whereas the sunshine strains are the simulated outcomes. The dimensions bar is 1 nm. f Summarized experimental and simulated FWHM of benzene rings alongside the lengthy axis in UiO-66-X. The experimental FWHM is the averaged worth obtained from greater than 20 cells, and the error bar is the usual deviation. Supply knowledge are supplied as a Supply Information file.
Theoretical explanations of various dynamic properties of UiO-66-X
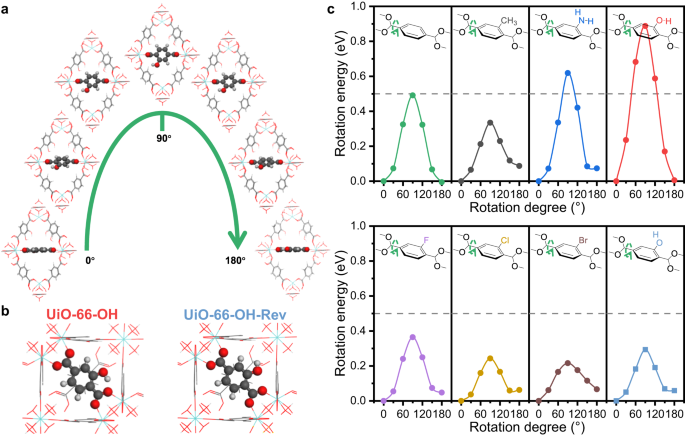
With the density useful idea (DFT), we calculated the rotation energies of BDC-X linkers and investigated the causes of various dynamic properties. The unique mannequin is constructed as a primitive cell of UiO-66-X, with the benzene ring perpendicular to the (110) airplane. The whole power of this mannequin is calculated because the power reference. The benzene ring of the chosen BDC-X linker is rotated with a sure diploma across the C2 axis, and the corresponding power is calculated with out ionic steps (Fig. 4a). The best power is all the time obtained when the benzene ring rotates by 90°, indicating that the state with benzene ring parallel to the (110) airplane is probably the most unstable (Fig. 4c). That is apparent as a result of two carboxyl teams and the benzene ring share a big π airplane, which is destroyed by rotation and 90° of π-flipping represents the least conjugation. The calculated activation power of π-flipping in UiO-66-H is 0.49 eV (47.4 kJ/mol), which is in step with the experimental ends in MOF-5 (47.3 kJ/mol)31.
a Schematic mannequin of benzene rotation in UiO-66-OH. b Schematic mannequin of two forms of UiO-66-OH, with completely different hydrogen course within the hydroxyl group of BDC-OH linkers. c The calculated rotation power of UiO-66-X towards the rotation diploma of benzene ring. The gray, crimson and white atoms signify C, O and H parts, respectively. Supply knowledge are supplied as a Supply Information file.
Nonetheless, completely different useful teams present completely different affect on the π-flipping properties. The rotation power at 90° can symbolize the issue of benzene rotation, the place a better power represents harder π-flipping and extra rigidity. Amino and hydroxyl teams present a stabilizing impact on the BDC-X linkers, whereas the opposite useful teams all improve the flexibleness (Fig. 4c). That is attributed to the intramolecular hydrogen bond of BDC-NH2 and BDC-OH (Supplementary Fig. 10). The space between hydroxyl H and carboxyl O in BDC-OH is 1.64 Å, and the bond size between amino H and carboxyl O in BDC-NH2 is 1.88 Å (Supplementary Desk 2). Quite the opposite, the gap between methyl H and carboxyl O in BDC-CH3 is for much longer (2.37 Å), indicating the existence of hydrogen bonds in UiO-66-OH and UiO-66-NH2, however not in UiO-66-CH3. The density of states (DOS) and crystal orbital Hamilton inhabitants (COHP) outcomes additionally verified the existence of hydrogen bonds (Supplementary Fig. 11). The optimistic worth of –COHP represents the bonding impact, whereas the unfavorable worth of –COHP represents the antibonding impact46. The built-in worth (–ICOHP) on the Fermi degree represents the general bonding situation. This worth follows the development of -OH…OOC > -NH2…OOC > -CH3…OOC, indicating that the power of intramolecular hydrogen bond follows the identical development. This explains why UiO-66-OH is probably the most inflexible pattern, the place the benzene can hardly rotate at RT. We construct a digital mannequin of UiO-66-OH-Rev by altering the orientation of hydroxyl H in BDC-OH (Fig. 4b). The intramolecular hydrogen bond will likely be destroyed, and the rotation power at 90° considerably decreases from 0.89 eV in UiO-66-OH to 0.29 eV in UiO-66-OH-Rev, indicating a better flexibility (Fig. 4c).
Due to this fact, the addition of useful teams, both electron donating (e.g. -CH3) or electron withdrawing (e.g. -F, -Br), will destabilize the BDC-X linkers, as a result of breaking of the symmetry of the conjugated construction. The benzene rings in BDC-X linkers will likely be extra rotatable, and UiO-66-X samples present extra flexibility. Nonetheless, -NH2 and -OH teams are the exceptions, as a result of they kind intramolecular hydrogen bonds, which overcomes the destabilizing results of functionalization and even inhibits the π-flipping. Thus, the UiO-66-NH2 and UiO-66-OH samples present native rigidity at RT, and the BDC-X linkers change into extra identifiable underneath electron beam in iDPC-STEM photographs.
Resolving the benzene rotation in UiO-66-X
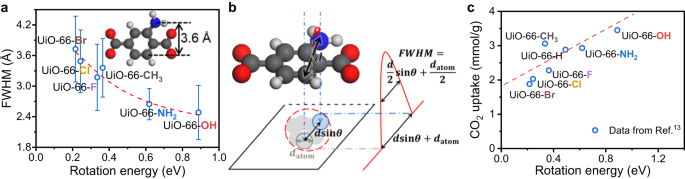
The calculated rotation power at 90° can also be in step with the FWHM obtained from iDPC-STEM. Increased rotation power corresponds to smaller FWHM, indicating a extra inflexible BDC-X linker (Fig. 5a). We will additionally match the experimental knowledge utilizing ({{{{{rm{FWHM}}}}}}=frac{d}{2}{{sin }}left(frac{{E}_{0}}{{E}_{R}}occasions frac{pi }{2}proper)+frac{{d}_{{{{{{rm{atom}}}}}}}}{2}), the place d represents the width of BDC-X linkers and ({E}_{R}) represents the rotation power at 90°. If the rotation diploma of benzene ring (θ) is 0, the BDC-X linker alongside the brief axis is parallel to the electron beam from [110] projection (Fig. 1a), as mentioned at the start. The BDC-X linker is then projected to a line, however nonetheless with a width. We assume this broaden impact as a relentless of ({d}_{{{{{{rm{atom}}}}}}}). If the benzene ring rotates with a level of θ, the width of BDC-X linkers is projected to (d{{sin }}theta). Then the projected vibrant spot of benzene ring ought to have a diameter of (d{{sin }}theta+{d}_{{{{{{rm{atom}}}}}}}). The depth profile alongside the lengthy axis ought to include a peak within the center, with FWHM equals to (frac{d}{2}{{sin }}theta+frac{{d}_{{{{{{rm{atom}}}}}}}}{2}) (Fig. 5b). We assume that the rotation of benzene ring originates from thermal movement, and the power ({E}_{0}) is mainly fixed at RT. The common rotation diploma might be written as (theta=frac{pi }{2}frac{{E}_{0}}{{E}_{R}}), the place we additional assume that the rotation power is mainly proportional to the rotation diploma.
a Fitted FWHM towards rotation energies. The fitted knowledge are measured with UiO-66-X (X ≠ H), and the correlation is ({{{{{rm{FWHM}}}}}}/{{{{{textual content{AA }}}}}}=1.89times {{sin }}left(frac{0.18,{{{{{rm{eV}}}}}}}{{E}_{{{{{{rm{Rotate}}}}}}}}occasions frac{pi }{2}proper)+1.82). The corresponding R2 is 0.96. The values of FWHM and ({E}_{{{{{{rm{Rotate}}}}}}}) are obtained from iDPC-STEM photographs and DFT calculations, whereas different parameters of d, ({E}_{0}) and ({d}_{{{{{{rm{atom}}}}}}}) are fitted. b Schematic mannequin of the imaging of BDC-X linkers. c Correlation between CO2 uptake and the rigidity of UiO-66-X. The values of CO2 uptake are from Ref. 13 and the adsorption situations are 1 bar and 0 oC. Supply knowledge are supplied as a Supply Information file.
Due to this fact, we will match the connection between FWHM obtained from iDPC-STEM experiments towards the rotation power at 90° calculated utilizing DFT (Fig. 5a). The becoming parameters are d, ({E}_{0}) and ({d}_{{{{{{rm{atom}}}}}}}) (Supplementary Desk 3). The fitted ({E}_{0}) equals to 0.18 eV (~17 kJ/mol), which is an inexpensive worth of thermal movement power at RT. For BDC-X (X ≠ H) linkers, the width is mainly the identical starting from 3.59 to 4.08 Å, with a mean worth of three.76 Å. The fitted worth of d is 3.78 Å, which may be very near the theoretical worth. The consistency of experiments and calculations additional affirm the reliability of our work.
Utilizing the fitted parameter of ({E}_{0}), we will calculate the common rotation diploma of benzene rings in UiO-66-X (Supplementary Desk 4). The simulated photographs of iDPC-STEM are then modeled utilizing these rotation levels. The simulated FWHM is in step with the experimental knowledge, indicating that the broaden of iDPC-STEM indicators originates from the rotation of benzene rings (Fig. 3f).
Correlating the native rigidity with the CO2 seize
The substitute of useful teams in UiO-66-X can strongly have an effect on the rotation properties after which native rigidity of those samples. The completely different macroscopic properties of the UiO-66-X samples with numerous useful teams are normally attributed to the static digital results24,25, beforehand. Nonetheless, the completely different dynamic properties also can considerably affect the macroscopic properties47,48, the research of which remains to be very inadequate. Impressed by the truth that CO2 adsorption can promote the flexibleness of MOFs49, right here we briefly focus on the potential impacts of UiO-66-X on CO2 seize, whose functionality is very associated to their native rigidity.
Utilizing the CO2 uptake knowledge from ref. 13, we will plot the connection between CO2 seize functionality and the rotation properties of UiO-66-X (Fig. 5c). The distinction in dynamic properties results in completely different CO2 seize efficiency, however their relationship has hardly ever been studied. Rigidity is taken into account useful to environment friendly assortment of visitor molecules, as a result of bigger variety of similar porous items48. Our outcomes agree with this view, and probably the most inflexible UiO-66-OH pattern has the very best CO2 uptake quantity. Though CO2 is taken into account as acidic and has a quadrupole second, probably the most primary UiO-66-NH2 or probably the most polarizable UiO-66-Br didn’t present the very best CO2 adsorption50 (Supplementary Fig. 12). As a substitute, the extra inflexible of the UiO-66-X pattern is, the extra CO2 it could possibly adsorb usually. The CO2 adsorption knowledge from different researchers51 are additionally in step with this development, though absolutely the worth of adsorption capability is completely different (Supplementary Fig. 13). These outcomes point out that functionalization can affect the macroscopic properties, not solely by tuning the digital construction, but additionally by altering the dynamic properties. Furthermore, when doping BDC-NH2 to UiO-66-H, a better fraction of BDC-NH2 linkers results in bigger CO2 uptake52, which can also be in step with the development that greater rigidity have a tendency to profit the adsorption of visitor molecules. Nonetheless, additional investigation is required to raised perceive the mechanism of how native rigidity influences the CO2 seize, and in situ inelastic- and quasi-elastic neutron scattering is likely one of the most correct methods53.
In conclusion, we studied the dynamic affect of useful teams on UiO-66-X samples. Utilizing iDPC-STEM know-how, we’re in a position to straight ‘see’ the rotation properties of benzene rings in BDC-X linkers, and the rigidity towards π-flipping is very associated to the useful teams. To the very best of our data that is the primary use of electron microscopy to picture the rotation properties of natural linkers in MOFs, which is a crucial complement to the spectral technique and gives an strategy to know the native flexibility of MOFs from a extra direct perspective. Among the many UiO-66-X samples, UiO-66-OH pattern exhibits the very best native rigidity, which is attributed to the robust intramolecular hydrogen bond. The benzene rings in UiO-66-OH and UiO-66-NH2 confirmed mainly the identical orientation at RT, which has not been reported. Furthermore, the distinction within the dynamic properties could be answerable for the completely different macroscopic properties, and we observe a optimistic relationship between CO2 uptake and native rigidity of UiO-66-X. These outcomes of assorted rotation properties of UiO-66-X pave the best way for his or her potential functions in capturing of small molecules, separation of natural compounds and molecular machines.
[ad_2]