[ad_1]
MD simulation
Impact of demulsifier intrinsic options
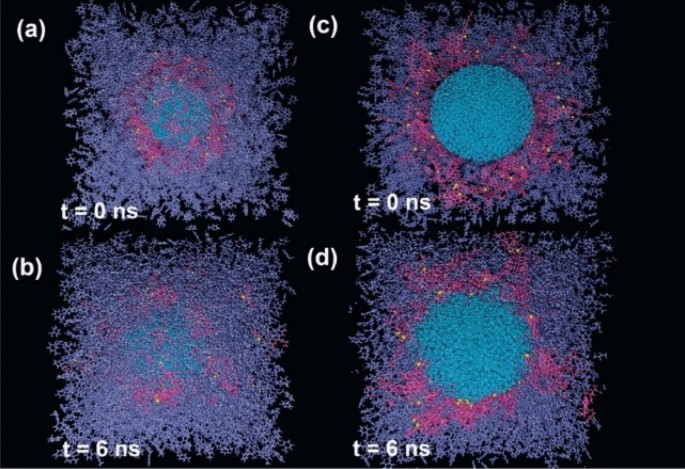
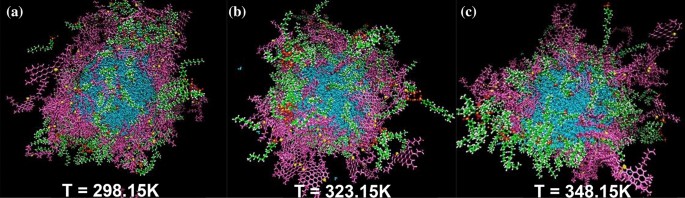
As a primary step, to search out out concerning the dimension of interfacial ASP movie, a number of ASP molecules had been put across the water droplet (Fig. 2a,c). After the calculation, the organized interfacial movie stemmed from ASPs adsorption signifies self-aggregation amongst ASPs at some elements4 (Fig. 2b,d). Typically, asphaltenes are likely to self-aggregate, this habits has additionally been seen in different techniques containing asphaltenes28. If the interfacial movie settled between two phases is assumed as a 2D movie, a fence-like construction seems. Nevertheless, an totally uniform movie was not noticed. The result’s in keeping with the outcomes authorized by the Cadena-Nava RD crew29. By characterizing 2D and 3D constructions within the ASP accumulations, they revealed the size of the interfacial movie and highlighted their significance.
The snapshot of the preliminary configuration of the system containing all contents apart from surfactant molecule (demulsifier) (a), and its facet view (c). The ultimate construction of the system (b), and its facet view (c). Colour code: colour schemes are the identical as Fig. 1.
Within the following, anionic and cationic surfactants as demulsifiers are individually added across the interfacial movie obtained from the earlier step to find out what occurs to the interfacial movie after publicity to varied demulsifiers. The interfacial movie options associated to demulsifier-added techniques had been in contrast with the movie options with none demulsifiers. Density is without doubt one of the important bodily portions to depict the system content material distribution30,31. Subsequently, the density profile within the course of the Z-axis is utilized to find out the transformation of interfacial ASP movie primarily based on the contents distribution variation20,32.
The noticed plots within the density of water (Fig. 3b–e) are acceptable proof to approve that the spherical construction of water droplets remained reasonably steady. By taking a look at Determine S2, it may be seen that by including surfactants to techniques, the density figures deviate from the figures of the non-surfactant system. This variation reveals that ASP movie has gone by a rearrangement5, thereby inflicting uncovered elements onto the floor of the water droplets. Underlying causes of showing asp-uncovered a part of droplets are the discount of the ASP molecule distance and extra aggregation on the place of most fluctuation33. In accordance with the density profiles (Fig. 3c–e), the cationic surfactant, CTAB, did it higher than others. However within the following, will probably be revealed why the CTAB molecule isn’t as efficient as anionic demulsifiers.
The snapshot of the ultimate construction of 4 techniques with non-surfactant, CTAB, SDS, SDBSand as demulsifier, from left to proper (a). Colour code: colour schemes are the identical as Fig. 1. The density profile of some contents of the 4 talked about techniques (b–e).
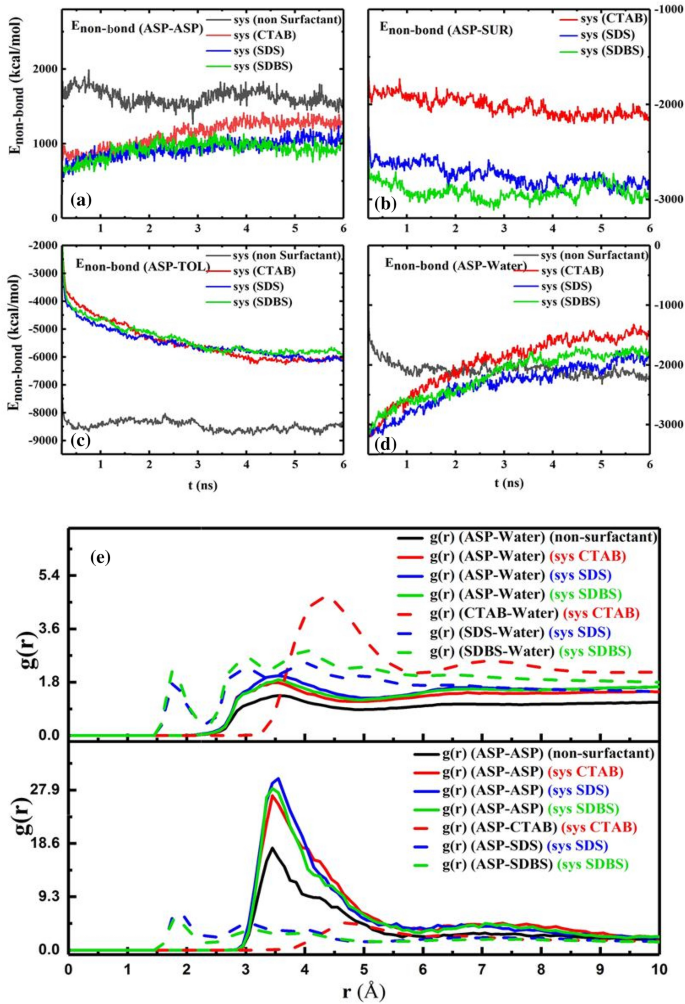
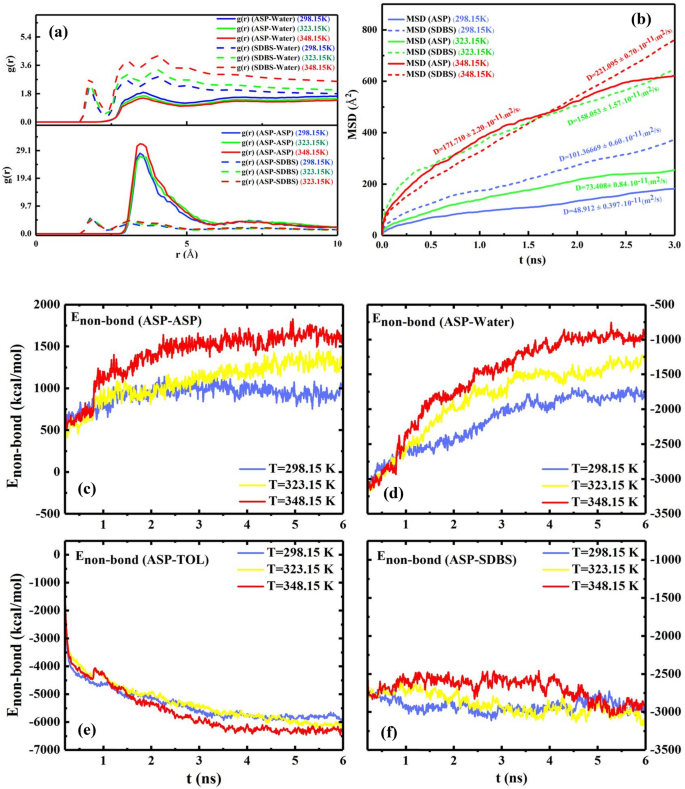
The snapshot of the water droplet (Fig. 3a) reveals that the demulsifiers make the water droplet uncover and the interfacial movie rupture extra34, concurrently. All modifications within the ASP movie are thought of movie transformations as an alternative of a movie eradication. Different bodily portions equivalent to non-bond power35 (Enon-bond), radial distribution perform18 (RDF or g(r)), imply sq. displacement (MSD), and diffusion coefficient (D)4 had been calculated to estimate the effectivity of demulsifiers. The Enon-bond is split into two elements:, specifically van der Waals interplay power (EVDW) and electrostatic interplay power (Eele). The contribution of every time period impacts the Enon-bond. The Enon-bond between pair contents is used to extra precisely predict the movie alteration (Fig. 4a–d). Within the presence of the demulsifier, the worth of absolute Enon-bond between ASP–ASP decreases, and likewise the gap between them declines. The change in distance will probably be indicated by the g(r) depth variation within the following. The contribution of the Enon-bond is proven in Determine S3. It’s noticeable that the extra absolutely the Enon-bond between the ASP–SUR, the much less absolutely the Enon-bond between the ASP-ASP molecules can be. This reverse development is kind of observable in Fig. 4a,b. Determine 4b depicts the next attraction power between the anionic surfactants and the ASP molecules, displaying the higher tendency of the anionic surfactants to be close to the ASP molecules. Additionally, by including the surfactants to the techniques, the Enon-bond of the ASP molecules with each oil and water section decreases Fig. 4c,d. The redial distribution perform (g(r)) reveals the gap of the shell surrounding a sure molecule36.
Determine 4e reveals that by embedding surfactants into the interfacial movie, the depth of the primary peak of g(r)ASP-ASP, at (r=) 3.45 Å, there’s a lower within the distance of ASPs18,37, and the primary peak of g(r)ASP-SUR corresponds to the hydrogen bonding calculated within the quantum part. The quantity of g(r) of CTAB-ASP signifies that the gap between CTAB and ASP molecule is bigger than the gap between the anionic surfactant and ASP38. So, the primary cause for the completely different efficiency of cationic and anionic surfactants will be traced to the distinction of their tendency to pick out adsorption websites on the interfacial movie. All the outcomes will be justified by the tendency of anionic surfactants’ head group to be adsorbed subsequent to the positively charged hydrogen bonded to the ASP heteroatom, i.e., N atom. These outcomes are in keeping with each Enon-bond between the surfactants and the ASP molecules and the interplay power obtained within the quantum part.
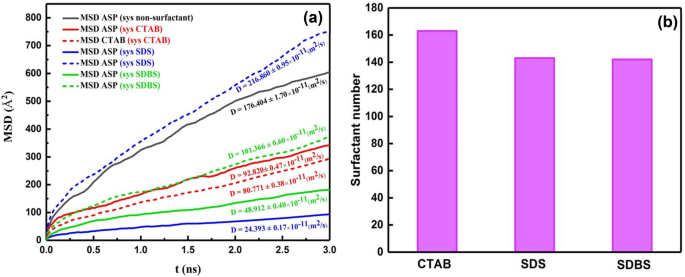
It was talked about above that demulsifiers had been added to the output of the primary calculation, which was the system with out demulsifiers (Fig. 2b,d), so after including the surfactant, the worth of MSD of ASPs reveals the displacement of the ASP molecules current within the ASP movie (Fig. 5a). Subsequently, the extra the ASP molecule displacement happens, the extra alteration will probably be noticed within the movie construction. Determine 5a depicts that probably the most worth of MSD and its slope39 (diffusion coefficient) belong to the system containing CTAB surfactant, which is in keeping with the density profile (Determine S1). It implies that probably the most variation associated to the density profile of ASP is in keeping with the development of MSD and diffusion coefficient within the system together with CTAB as a demulsifier. In accordance with these outcomes, it’s anticipated that the efficiency of the CTAB surfactant will probably be larger than the efficiency of anionic surfactants. However, as well as, the variety of adsorbed surfactants on the water floor can considerably predict which kind of surfactant will be extra environment friendly in high-yielding demulsification. Though CTAB molecules contribute to extra rearrangement of the movie, the variety of CTAB molecules adsorbed onto the water floor causes a circumstance that both seals or drains away the water droplets.
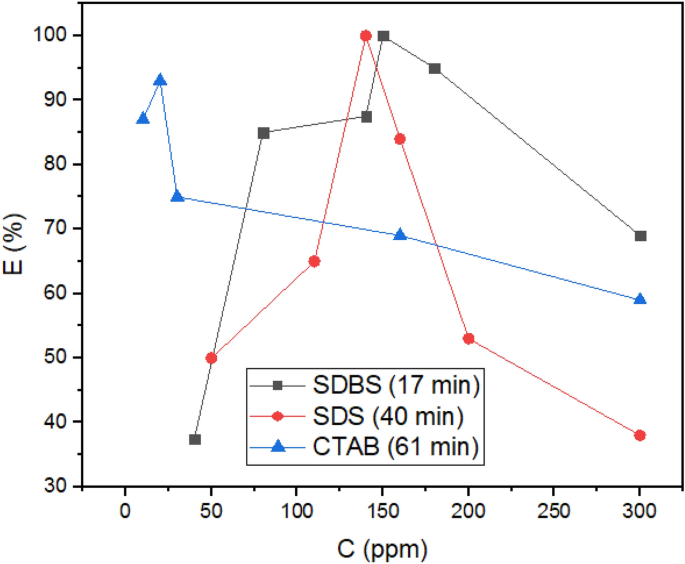
Determine 5b shows the extra CTAB molecules adsorbed on water. It’s defined that the extra the demulsifiers are adsorbed on the water droplet, the extra the rupture must be sealed. Subsequently, this phenomenon can constrain draining water away from the droplet to adjoining droplets and likewise hamper the formation of larger surrounded droplets in comparison with the case of anionic demulsifiers. Though g(r) peak positions (Fig. 4e) and Eint (Desk S3) of anionic surfactants present that they’ve shaped extra strong hydrogen bonds with water molecules compared to the cationic ones, Eadvert within the quantum part conveys that anionic surfactant need to be close to the ASP molecule. The size of the hydrogen bond was confirmed by the out there peak at (r) ≈ 2 Å (Fig. 4e) and Desk S4. Regardless of the extra strong hydrogen bond between anionic demulsifiers and water, the tendency of the anionic surfactant to be adsorbed close to the ASP molecule can forestall the water droplet from being sealed. The outcomes of this part solid a optimistic mild on the way in which of choosing simpler demulsifiers and might pave the way in which for anticipating that anionic surfactants, SDS and SDBS, will be extra sufficient demulsifiers than cationic surfactants (CTAB). As a result of not solely do they work together with the ASP molecules and remodel the interfacial movie, but additionally they make ruptures within the interfacial movie and protect them. All of those are conducive to creating a greater demulsification perform. As well as, among the many anionic surfactants, SDS and SDBS, SDBS has been thought of to be a extra environment friendly demulsifier due to the benzene ring in its construction. The π- π interplay between ASP and SDBS helps SDBS keep close to the ASP molecule. In accordance with the analysis investigation carried out by Ming Duan2 the efficient demulsifier tends to settle within the neighborhood of not solely water but additionally the ASP molecules. Subsequently, SDBS has been chosen as the specified surfactant, which was confirmed by the experimental outcomes obtained within the bottle take a look at (Fig. 12).
Noticed development of accelerating demulsifier focus
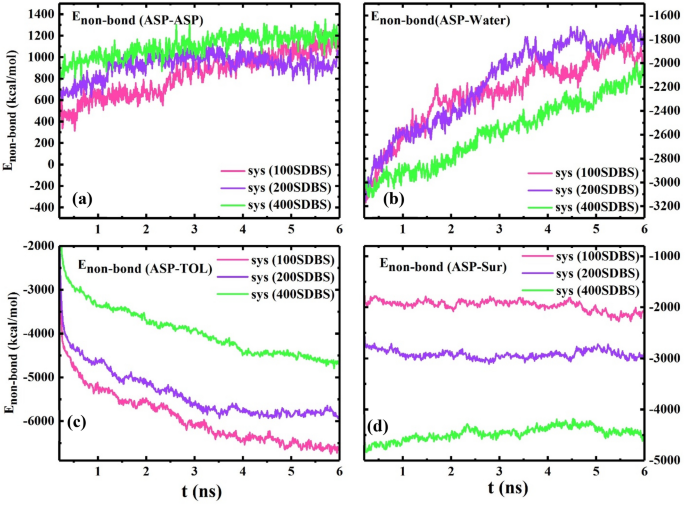
After choosing SDBS as the specified surfactant, the impact of the demulsifier focus on the interfacial movie was investigated. Determine 6a reveals the techniques consisting of 100, 200, and 400 SDBS surfactant molecules residing across the water droplets surrounded by ASPs. In accordance with the earlier part, the MSD worth was utilized to scrutinize the alteration within the ASP movie. Determine 6b reveals that the MSD (ASP) of the system (200 SDBS) is greater than that of others, which reveals that the ASP movie modifications extra, whereas 100 SDBS molecules aren’t propellant sufficient to trigger this variation.
The snapshot of techniques with 100, 200, and 400 ASP molecules, respectively (a). The imply squared displacement and its slope (diffusion coefficient) belonging to ASP and demulsifier molecules for the techniques (b). The RDF of the techniques with the completely different variety of demulsifier molecules (c).
However conversely, by redoubling the variety of surfactants, from 200 to 400 surfactants, the MSD worth decreases. It’s noticeable that after the required variety of demulsifiers, the interfacial movie construction undergoes fewer modifications relative to the preliminary construction. Along with the MSD plot, Determine S2 reveals that the ASP density figures show the extra ruptures prompted on the water floor (system 200 SDBS), and conversely, the extra steady association of ASP movie (system 400 SDBS), which is noticed from the much less fluctuation of the density profile.
For the system 400 SDBS, the Enon-bond between ASP-ASP molecules reaches the next degree (Fig. 7a), and each g(r)ASP-ASP and g(r)ASP-SUR intensities decline (Fig. 6c), that are acceptable proof for preserving the ASP movie construction by stopping its formation from altering. These promising outcomes can anticipate that past the required focus, at 400 SDBS, the surfactant molecules can be part of collectively and kind self-aggregation and consequently give rise to retaining the preliminary association of the ASP movie. Subsequently, at this focus, draining the water droplet away34 turns into much less, and the quantity of water extracted from the oil section will cut back. This prediction is in good settlement with the experimental lead to “Bottle take a look at“, displaying a degree wherein a reverse development happens.
The temperature impact on ASP movie
The earlier part examined the influence of the specified surfactant focus on the interfacial movie. The temperature effectivity on the interfacial movie is one unconsidered analysis within the earlier research of demulsification. Within the present a part of this examine, all system situations besides the temperature are equal. In accordance with the outcomes assessed in each earlier elements, a few of the figuring out components to foretell the advance of the demulsification development embody (a) growing depth of g(r) (ASP-ASP), (b) showing g(r) (ASP-SUR) in much less radius, (c) lowering Enon-bond between ASP-ASP molecules, (d) and enhancing the MSD of ASP molecules within the interfacial movie. Subsequently, it appears uncommon that by growing the temperature, absolutely the worth of Enon-bond among the many ASP molecules will increase (Fig. 8c–f) whereas the depth of g(r)ASP-ASP reaches the next degree (Fig. 8(a)). To analyze the surprising development, the outcomes of MSD turned extra important. Because the MSD plot reveals (Fig. 8b), the extra the system receives the temperature, the extra the ASP displacement happens. These displacements enable ASP molecules to undergo extra spatial orientations relative to one another. Mousavi40 and coworkers demonstrated the diagonal construction has much less distance.
The RDF of the techniques with 200 SDBS molecules, as demulsifier, at completely different temperatures of 298.15 Okay, 323.15 Okay, and 348.15 Okay (a). The imply squared displacement and its slope (diffusion coefficient) belonging to ASP and demulsifier molecules at completely different temperatures (b). The plot of ({E}_{non-bond}) between some pair contents of the techniques at completely different temperatures (c–f).
However, assuaging the depth of g(r)ASP-WAT is a chunk of proof of a reasonably declining interplay between water and the ASP molecules (Fig. 8a), which causes extra conformation modifications within the ASP molecules. As well as, the upper worth of surfactant’s MSD stemmed from growing temperature conforms to the next tempo of surfactant towards interfacial movie, which accelerates the remodeling of the interfacial movie (Fig. 9). The obtained outcomes advocate that the upper temperature a system has, the extra unstable the system turns into. Consequently, it turns into clear that extra water can be separated at the next temperature. The outcomes are in keeping with these of experimental investigations defined in “Figuring out the temperature position“, which confirms that extra water is extracted from the oil section.
Quantum calculation
A number of research have proven that the quantum calculation is an sufficient methodology to judge the adsorption of demulsifiers on ASP and to evaluate completely different interactions between them. Ren and his crew used the quantum calculation to estimate the interplay between graphene oxide and ASP16. Additionally, the DFT methodology was employed by Flores and coworkers to review the correlation between quantum parameters and the experimental habits of various demulsifiers41.
The adsorption of surfactants on ASP
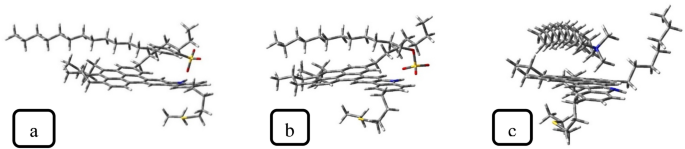
Desk 1 presents the adsorption power of surfactants on ASP (calculated utilizing Eq. 1). The adsorption energies of anionic surfactants are comparatively equal, whereas the adsorption power of CTAB is notably decrease than theirs. This development was confirmed by Eint calculated within the MD simulation (“Impact of demulsifier intrinsic options“). Usually, within the demulsification course of, the next adsorption power will be simpler as a result of it may be conducive to the transformation of the interfacial ASP movie. Furthermore, the SDBS adsorption configuration on ASP (Fig. 10) reveals that the SDBS benzene ring interacts with the fragrant ring of the ASP construction, which ends up from the π–π interplay. In accordance with some analysis investigations26,42, the π–π interplay between ASP and the demulsifier has optimistic results on the demulsification perform. In continuation of this part, some parameters influencing surfactant–ASP interactions will probably be evaluated.
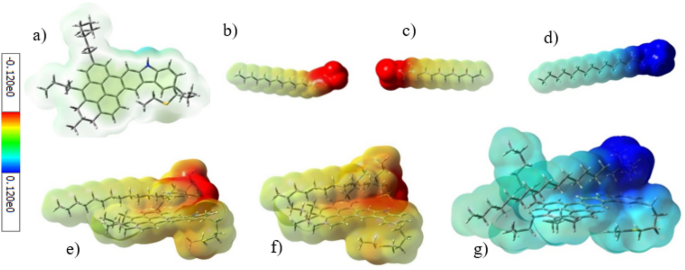
An electrostatic interplay, as one of many doable interactions between the surfactant and ASP, is attributed to each the web cost of ionic surfactants and completely different potential prices of ASP’s heteroatoms and practical teams. To analyze the electrostatic interplay, the electrostatic floor potential (ESP) was used. Determine 11 signifies the ESP for ASP and surfactants, with crimson and blue colours representing the detrimental and optimistic potential, respectively. Subsequently, the anionic and cationic surfactants have a detrimental and optimistic potential respectively, which is expounded to the detrimental and optimistic prices of their heads. Regardless of the impartial nature of ASP, each websites with a optimistic and detrimental potential will be seen on it concurrently. It’s noticeable that the blue colour close to the nitrogen atom refers back to the present optimistic cost, whereas the shortage of readability of the yellow colour reveals that the detrimental potential was distributed in lots of areas of the ASP construction.
The ASP website, containing a optimistic potential, is an appropriate place for the adsorption of anionic surfactants, on which the anionic surfactant heads had been adsorbed. As well as, Fig. 11 reveals the chnage within the ASP potential space, which stems from the robust interplay brought on by the adsorption of the surfactant. Opposite to the anionic surfactants, the CTAB surfactant head, containing a optimistic potential, can’t be adsorbed on this website due to the electrostatic repulsion. Thus, the electrostatic attraction between ASP and the anionic surfactants, SDBS and SDS, performs a key position in growing their absolute adsorption power at a sure distance.
The HOMO and LOMO energies had been calculated for some contents together with surfactants, ASP, and the advanced of an adsorbed surfactant on ASP. The digital properties of contents can help us to grasp the interplay between them. Determine S6 in SI reveals the graphical form of HOMO and LUMO for ASP, surfactants, and surfactants-ASP. Along with the shapes of HOMO and LUMO, the power hole is a crucial parameter calculated from the HOMO and the LUMO energies. The power hole of ASP-SDBS is 5.31 eV (Desk 1), which is decrease than the power hole of each ASP and SDBS individually. The discount of the power hole is an index for the interplay between ASP and SDBS43. This phenomenon was noticed for 2 different surfactants.
The worldwide hardness is one other parameter derived from the HOMO and LUMO power, which is an index for reactivity. Thus, a compound with the next power hole is tougher and fewer reactive25. Between the 2 anionic surfactants, SDBS with a decrease hardness worth turned extra polarized than SDS. So, through the interplay with ASP, SDBS was simpler than SDS. The digital chemical potential of various compounds, estimated utilizing the HOMO and LUMO energies, can decide the course of the electron switch. The ASP molecule has a decrease digital chemical potential than each of the anionic surfactants, so it conducts and transfers electrons in direction of ASP. This habits was authorized with a rise within the crimson space of ASP (Fig. 11). However, the decrease chemical potential of CTAB, in contrast with ASP, results in a reverse habits for CTAB.
A number of oxygen atoms out there within the head group of anionic surfactants allow the formation of hydrogen bonds (HB) between ASP and the anionic surfactants. The outcomes of the AIM evaluation reveal that one of many oxygen atoms out there in an anionic surfactant construction might kind the hydrogen bond with the hydrogen linked to nitrogen in ASP. By evaluating the cost density, ρ, and the bond power of HBs, it may be concluded that the HB energy between ASP and SDS is barely stronger than the HB energy between ASP and SDBS (Desk 2), which is confirmed by a shorter HB size. The HB size of anionic surfactants is constant whit the g(r) obtained within the MD simulation part (Fig. 4e). Additionally, it must be emphasised that HB formation between ASP and the demulsifiers can deform the ASP movies, thereby accelerating the demulsification course of. In accordance with these promising outcomes, it’s anticipated that each anionic surfactants would separate water extra effectively than CTAB.
Experimental part
Bottle take a look at
The scale of water droplets within the emulsion was estimated to be 2.3 ± 1.5 μm by optical microscope. A number of research have proven that the scale of water droplets within the water-in-oil emulsion can differ from a sub-micrometer44 to a a number of micrometers44.
The outcomes of the bottle take a look at for all surfactants (Fig. 12) point out that the anionic surfactants as demulsifiers are extra environment friendly than the cationic surfactant (CTAB). In accordance with the figures, SDBS and SDS utterly eliminated water from crude oil (at 17 and 40 min respectively), whereas CTAB can’t utterly dehydrate it (95% at 61 min). The quantity of water remaining in crude oil after demulsification by surfactant CTAB is greater than allowed45. This development was anticipated with MD and quantum calculations. On this approach, that anionic surfactants have stronger interactions with ASP (Desk 1; Fig. 4b). The interplay of demulsifiers with ASP modifications the ASP hydrophilicity46, thereby coagulating the water droplets extra quickly. The formation of HB for anionic surfactants, as one other issue calculated with AIM, propels the method of demulsification26. Additionally, hydrogen bonding can have an effect on asphaltene accumulation47. It must be identified that each one surfactants utilized on this examine can dehydrate crude oil by rupturing the ASP movie surrounding the water droplets. The modifications in Eint and g(r) associated to ASP-ASP, which had been computed as proof of movie modifications within the MD part, passed off following the addition of demulsifiers to crude oil. As proven in Fig. 1, SDBS has a benzene ring that facilitates its diffusion within the crude oil medium26, thus enhancing the SDBS perform compared to SDS. Moreover, the SDBS benzene ring interacts with ASP out there within the interfacial movie16 and helps to coalesce the person water droplets. These observations verify the important position of the benzene ring within the demulsification course of, which is consitent with some approving analysis concerning the constructive impact of the fragrant ring16,26,42.The effectivity of demulsification will be manipulated by one other parameter referred to as hydrophilic–lipophilic stability (HLB). The upper the HLB worth the surfactant has, the extra the surfactant is hydrophilic. The HLB worth for SDBS and SDS had been reported to be 10.6 and 40, respectively48. The SDS head with the next worth of HLB has extra tendency to be entrapped within the water section49, whereas the average HLB worth for SDBS helps to take care of its head on the water–crude oil interface, so it will enhance the demulsification perform. Varied components, which belong to the demulsifier nature, have an effect on the demulsification effectivity, however many papers have reported that the demulsifiers with average hydrophilicity perform higher49,50.
As talked about within the MD half (Fig. 6a), the sealing of ruptures by the additional surfactants takes place after an optimum focus, so it decreased the dehydration effectivity of the three surfactants. Electrostatic repulsion can have a fantastic affect on the habits of techniques containing ionic surfactants51, subsequently the electrostatic repulsion of the ionic surfactant on the interface is another excuse52. Based mostly on the MD outcomes indicating a higher variety of CTAB sealed out there ruptures on the interface (Fig. 5b), it’s anticipated that CTAB as a demulsifier would have a much less optimum focus. Moreover, SDS and SDBS with a decrease and comparable variety of adsorbed demulsifiers on ruptures ought to have an equal optimum focus, i.e., larger than the CTAB optimum focus. All predictions concerning the optimum focus had been confirmed by the experimental method, i.e., the optimum concentrations for CTAB, SDS and SDBS had been 20, 140, and 150 ppm, respectively (Fig. 12). The lower within the dehydration effectivity after the optimum focus has been reported in some research26,53,54.
Determine 12 signifies that past the required focus which belong to all the supposed demulsifiers, the separated water decreses. There are two reasonsfor the phenomenon talked about above, specifically (1) the electrostatic repulsions between similar demulsifiers, and (2) the sealing movie of the demulsifier surrounding the ASP interfacial movie. These phenomena are widespread within the demulsification course of and have been reported in some research26,55.
Figuring out the temperature position
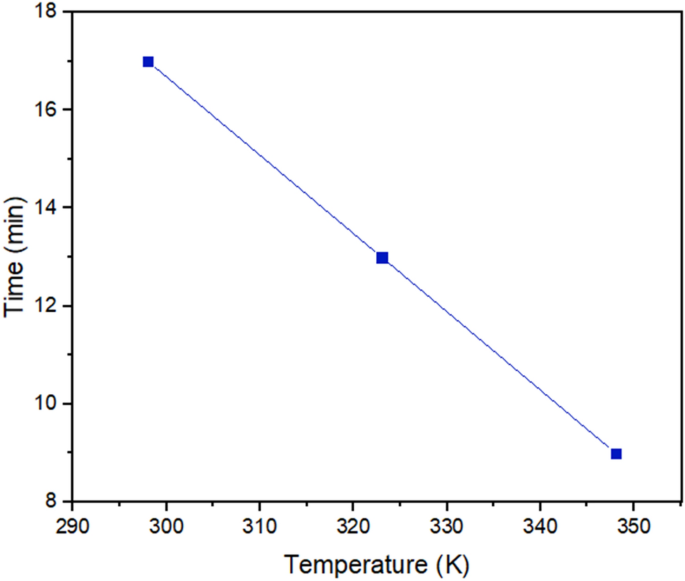
To check how temperature impacts the demulsification course of, the SDBS optimum focus was thought to be the specified focus, and the temperature vary was thought of 298, 323, and 348 Okay (Fig. 13). Some research have demonstrated the significance of the temperature impact on the demulsification course of26,56,57. The bottle take a look at outcomes defined that the demulsification time for the whole removing of water from crude oil declined by growing the temperature. The extra the temperature of the system is, the higher the demulsification course of perform can be. It’s time to scrutinize the underlying components behind the temperature as a propellant issue. The discount in viscosity brought on by growing the temperature is named a major issue within the enchancment of demulsification efficiency58 as a result of on this state of affairs, water droplets might diffuse collectively simply. Moreover, growing the temperature not solely accelerates the motion of demulsifiers in direction of the interface26, but additionally can improve the kinetic power of water droplets and the collision between them59. The MSD outcomes displayed in Fig. 8b verify the rise in motion of the the demulsifier together with the rise within the temperature.
The soundness of the ASP movie across the water droplet can change with temperature modifications60, the MD outcomes talked about above predicted that the displacement of ASP will increase with the rise in temperature. So, it’s extra prone to alter the interface movie and extract the water effectively. On this approach, the water droplets coalesce shortly. This development was confirmed by the bottle take a look at on this part.
[ad_2]