
[ad_1]
A Press Launch from Improvement
Infertility impacts round 48 million {couples} worldwide and may have varied causes. In mammals, together with people, eggs are produced within the ovary. When this course of goes improper, it may possibly result in feminine infertility. One instance of that is untimely ovarian insufficiency, which is characterised by issues with egg manufacturing earlier than the age of 40. As much as 3.7% of females expertise infertility on account of this situation, and round 30% of instances are as a result of genetic variations. Professor Kehkooi Kee, from Tsinghua College, China, who helped lead the examine, has been investigating this situation for a number of years. “In 2019, our collaborators, Professor Li’s crew, encountered a household with untimely ovarian insufficiency during which adjustments to a gene referred to as Eif4enif1 gave the impression to be accountable for the illness,” stated Professor Kee. The researchers determined to breed this genetic change in mice to attempt to perceive the way it impacts human infertility. They present that the eggs of those mice are affected by adjustments to their mitochondria – the powerhouses of the cell – and publish this new discovery within the journal Improvement on 13 December 2023.
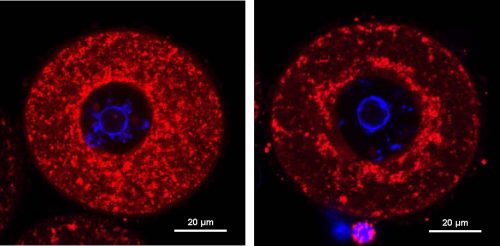
The researchers used CRISPR to introduce the genetic change within the mice. They allowed these mice to develop up after which in contrast their fertility with the fertility of mice whose DNA had not been edited. Yuxi Ding, the primary creator and a MD/PhD scholar who led the examine, discovered that the typical variety of whole follicles (the tiny sacs that include growing eggs) was diminished by roughly 40% in older and genetically edited mice (the typical pup quantity in each litter was diminished by 33%. Importantly, when grown in a dish, about half of the eggs that had been fertilised didn’t survive past the early phases of growth. This demonstrated that, similar to the human sufferers, these mice had been experiencing issues with fertility.
When the researchers studied the eggs from these mice beneath the microscope, they observed one thing uncommon about their mitochondria. Mitochondria produce the power that cells, together with egg cells, want. Mitochondria are normally evenly distributed all through the egg, however the mitochondria in eggs from mice with the genetic variation had been clustered collectively. “We had been truly stunned by the variations within the mitochondria,” stated Professor Kee. “On the time we had been doing this analysis, a hyperlink between Eif4enif1 and mitochondria had not been seen earlier than.”
It appears seemingly that these misbehaving mitochondria are contributing to the fertility issues in these mice, main the researchers to suggest that restoring correct mitochondrial behaviour may enhance fertility. This examine gives course for future analysis in human infertility, reminiscent of establishing whether or not mitochondrial defects are additionally discovered within the eggs of human sufferers with untimely ovarian insufficiency and whether or not these identical mitochondrial defects are noticed in embryos after the eggs are fertilised. As well as, testing whether or not restoring the conventional distribution of mitochondria improves fertility may turn out to be a brand new therapy technique. “Our analysis means that rescuing oocyte mitochondria abnormality may very well be a possible therapeutic goal for scientific infertility sufferers with genetic variants,” says Professor Kee.
Ding, Y., He, Z., Sha, Y., Kee, Okay., Li, L. (2023). Eif4enif1 haploinsufficiency disrupts oocyte mitochondrial dynamics and results in subfertility. Improvement, 150, dev202151. doi: 10.1242/dev.202151
[ad_2]
 (No Rankings But)
(No Rankings But)