
[ad_1]
A while in the past in 2010, I confirmed a chemical drawback I used to set throughout college entrance interviews. It was all about sample recognition and the way one can develop a speculation primarily based on this. In that occasion, it concerned recognising {that a} cyclic molecule which appeared to have the cyclohexatriene benzene-aromatic sample 1 was the truth is a trimer of carbon dioxide. Maybe small quantities of this fragrant molecule exist in options of fizzy drinks? Analysing these patterns occupied about 10-20 minutes of an interview, and though you may suppose I used to be posing a troublesome problem, many college students efficiently rose to it! Now I revisit, however with a barely higher actuality test on a associated molecule 2 (cyanuric acid).
As many as 58 examples of crystal buildings of 1,3,5-triazinane-2,4,6-trione 2 (cyanuric acid) are identified, usually with a co-adduct. Cyanuric acid is in impact a cyclic trimer of isocyanic acid moderately than of carbon dioxide. These examples are usually planar, with a imply C-N ring distance of ~1.37Å and a C-O distance of 1.22Å.
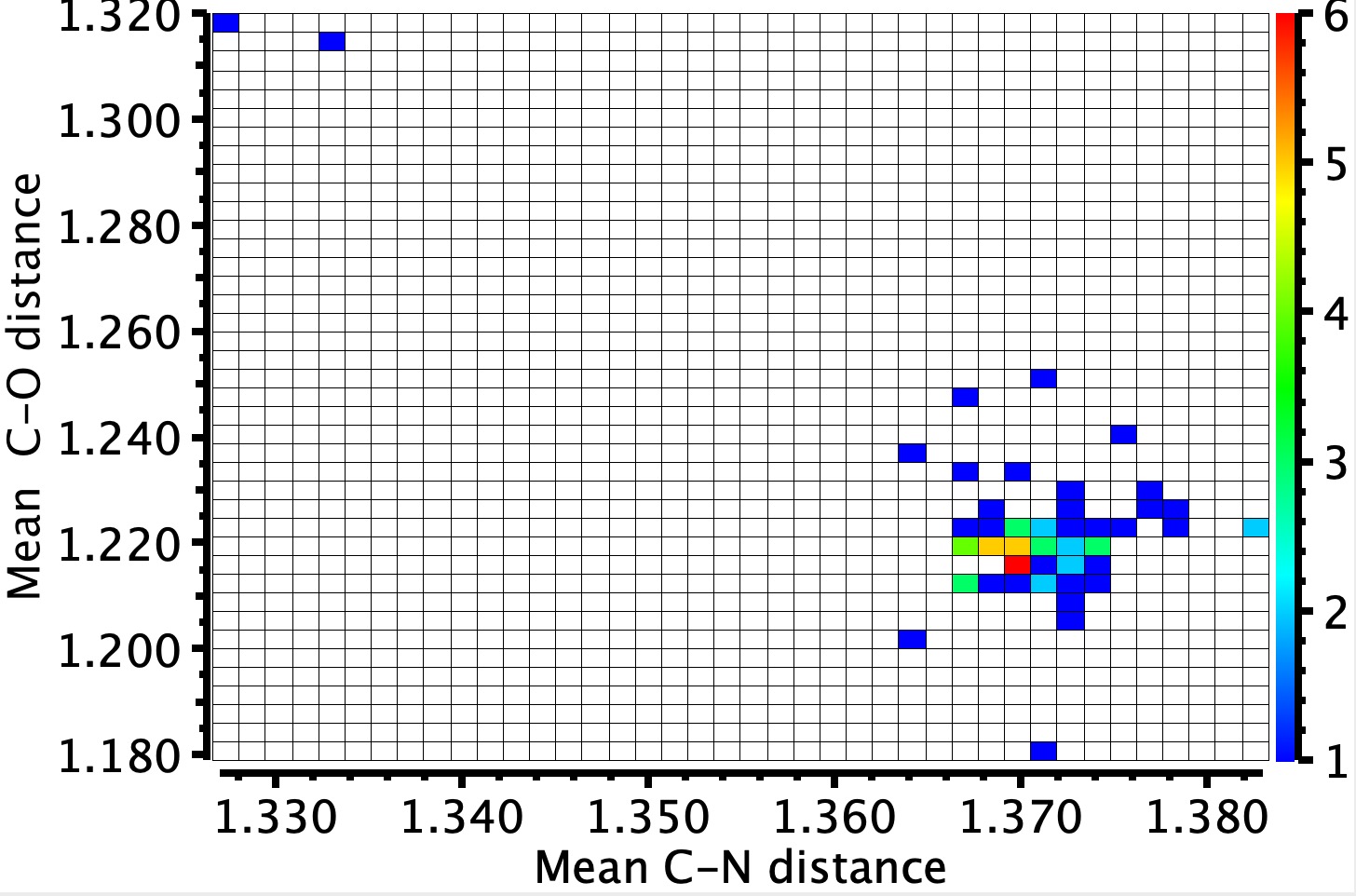
Two outliers stand out, each from a really lately printed article, being a co-adduct with melamine (1,3,5-triazine-2,4,6-triamine).[1] QACSUI02 reveals a shorter C-N distance of ~1.33Å however an extended C-O distances of 1.32Å and have a symmetrical patten of hydrogen bonds to the six receptors of the central unit. May this correspond extra carefully to the cyclohexatriene resonance buildings proven to the left of the diagram on the high? The primary process is to see if these bond lengths may be replicated utilizing calculation (usually a helpful process to test that the crystal construction is appropriate). For this goal, the construction beneath was chosen as the place to begin for varied fashions, utilizing an ωB97XD/Def2-TZVPP mannequin.
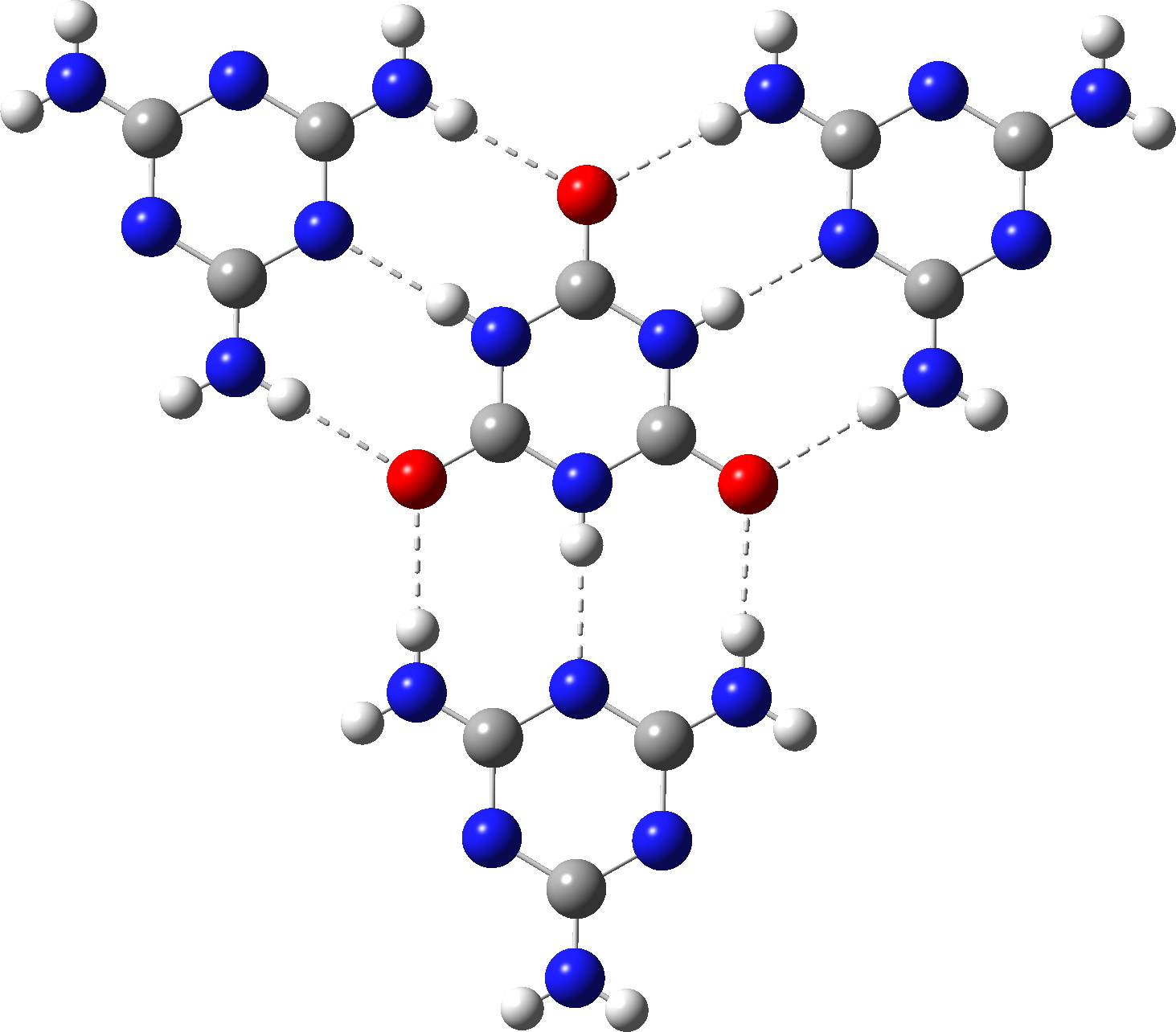
This creates a thriller. The calculated bond lengths present that while H-bonding to the central ring decreases the C-N size by 0.014Å and will increase the C-O size by 0.017Å, this impact is nowhere close to giant sufficient to match the obvious lengths within the crystal construction, the place a C-N impact of ~0.037Å can be wanted.
One other system XAKSOU has been reported the place discrete LiCl items substitute the hydrogen the H-bonds fashioned to melamine above.[2] A Li is coordinated to the carbonyl oxygen as a substitute of a hydrogen bond, and a chloride anion from one other molecule within the unit cell replaces the H-bond to nitrogen.
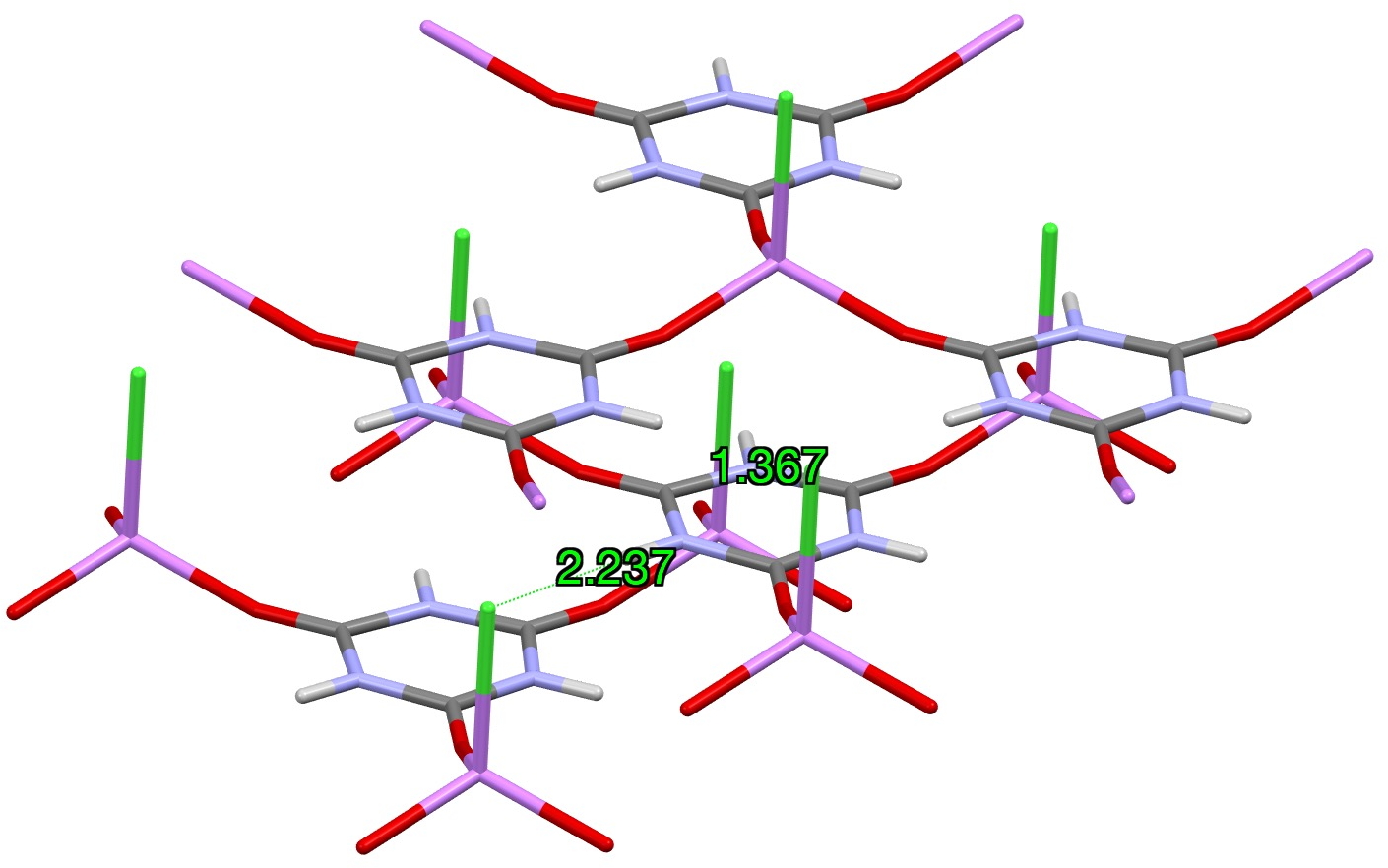
Within the computed mannequin, an intramolecular Cl-H hydrogen bond is used because the mannequin, leading to comparable C-N lengths because the crystal construction (one which doesn’t match the lengths within the outlying crystal construction QACSUI02)
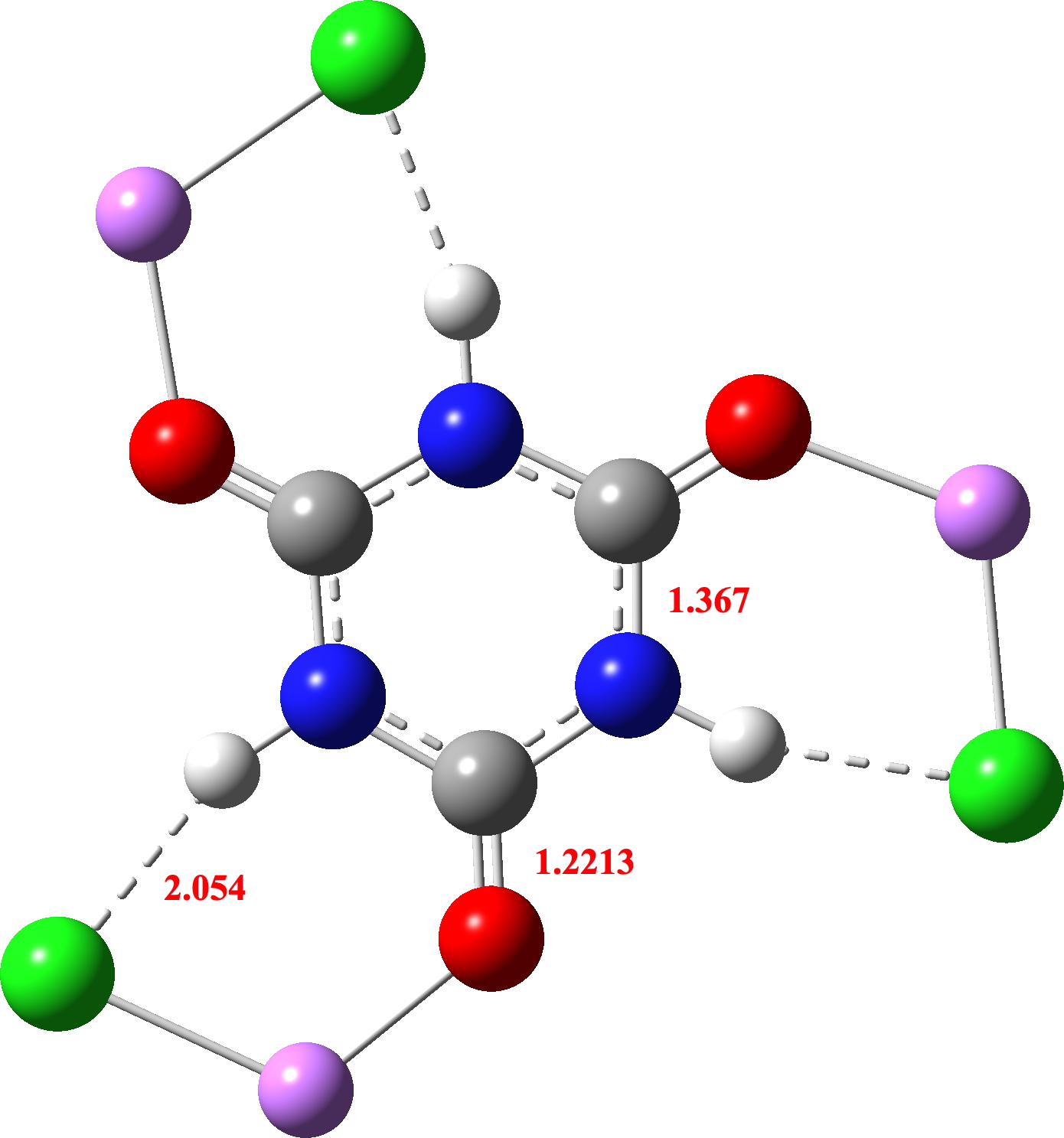
So the ultimate query to ask is whether or not this latter construction is fragrant. NICS(0)/(1) values of -2.8/-1.1ppm are computed, which suggests little or no aromaticity (fragrant values can be -10 to -20 pm). So it doesn’t appear as if aromaticity may be tuned into cyanuric acid 2 by polarising each the NH and CO items with ionic/H-bond interactions in order that the fragrant cyclohexatriene motif is best favoured over the 1,3,5-triazinane-2,4,6-trione non-aromatic resonance type. Are there some other examples the place aromatically tunable molecules is perhaps doable?
[ad_2]