
[ad_1]
It is a venerable natural response, which curiously I’ve not beforehand coated right here. First described in 1859, its nature was solely correctly elucidated in 1873. It’s a member of a category of response I’ve beforehand named “solvolytically assisted pericyclic”, or “perisolvolytic“. Right here I discover among the refined stereoelectronic results noticed for this apparently easy response.
It applies to a category of molecule generally known as 1,2-diols. Protonation is shortly adopted by migration of a (on this instance) methyl group, adopted by deprotonation of the carbonyl group fashioned by this course of. There are two mechanistic levels, the primary being the departure of the now protonated “ol” unit, and the second the migration of the methyl. In most textual content books and naturally Wikipedia, these are proven as very distinct steps. However they might additionally happen in a single concerted step, albeit most likely asynchronously.
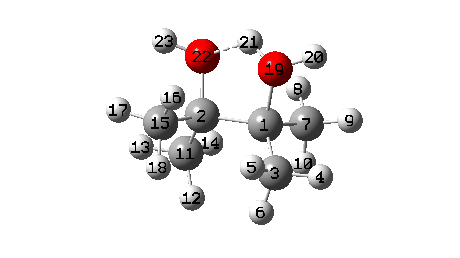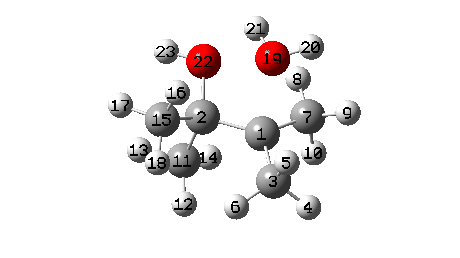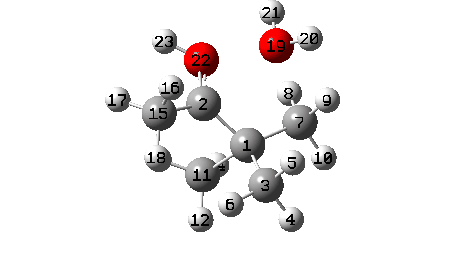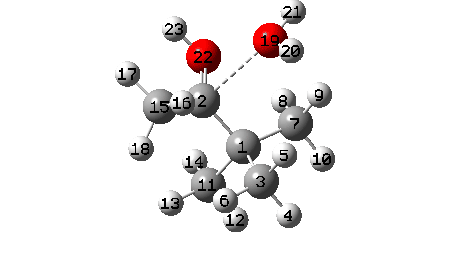
A B3LYP+GD3+BJ/Def2-TZVPP/SCRF=ethanol calculation offers mechanistic element (FAIR Information 10.14469/hpc/1769)
- To begin with, we be aware the H-bond fashioned between O22-H21. Between IRC = -10 and -6, this lengthens from 1.625Å to 1.843Å, destabilising the protonated alcohol group.
- Between IRC -6 to -1, the C1-O19 bond breaks, from a beginning size of 1.556Å to ~2.787Å.
- When IRC 0.0 is reached (the transition state), the C11 methyl begins emigrate throughout, a course of principally full by IRC +2.
- The ultimate stage is formation of a weak interplay between C2 and O19 to achieve IRC 7.
- A number of extra minor results can be discerned. Firstly methyl C3 rotates, to arrange a greater hyperconjugative interplay with the momentary carbocation forming at C1. This rotamer types the primary of a number of “hidden intermediates” within the response, intermediates which nearly kind earlier than being consumed, at IRC -6.5 (see the plot above labelled RMS gradient kind, for the minimal within the operate at this IRC worth).
- One other hidden intermediate seems at IRC -2, being the transient carbocation, as proven in stepwise variations of this mechanism, such because the Wikipedia web page. However its not actual, merely hidden! Because it approaches, methyl C7 rotates to maximise the hyperconjugative interactions.
- At IRC ~+3, methyl C15 rotates to once more maximise hyperconjugation with the newly fashioned C=O bond.
Ca we quantify a few of these results? This may be achieved by computing localised orbitals (NBOs) and pairwise interactions between a donor NBO (a bond or a lone pair) and an acceptor NBO (an antibonding orbital).
- The E(2) interplay between donor bond C2-C11 and acceptor C1-O19 is 3.3 kcal/mol (above the noise, however not particularly sturdy). It corresponds to an antiperiplanar alignment of the C2-C11 σ orbital and the C1-O19 σ* orbitals and ends in the breaking of bond C2-C11 (and reformation as C1-C11).
- The E(2) worth between donor lone pair O22 and acceptor C2-C11 σ* is 6.9 kcal/mol and corresponds to antiperiplanar alignment of those two orbitals, leading to formation of the C=O carbonyl π-bond, while concurrently rising the antibonding character of the C-C bond to encourage it to interrupt.
Fashions of those two interactions will be seen under. Click on on the picture to load them. The color blue overlaps positively with the color purple, and crimson with orange.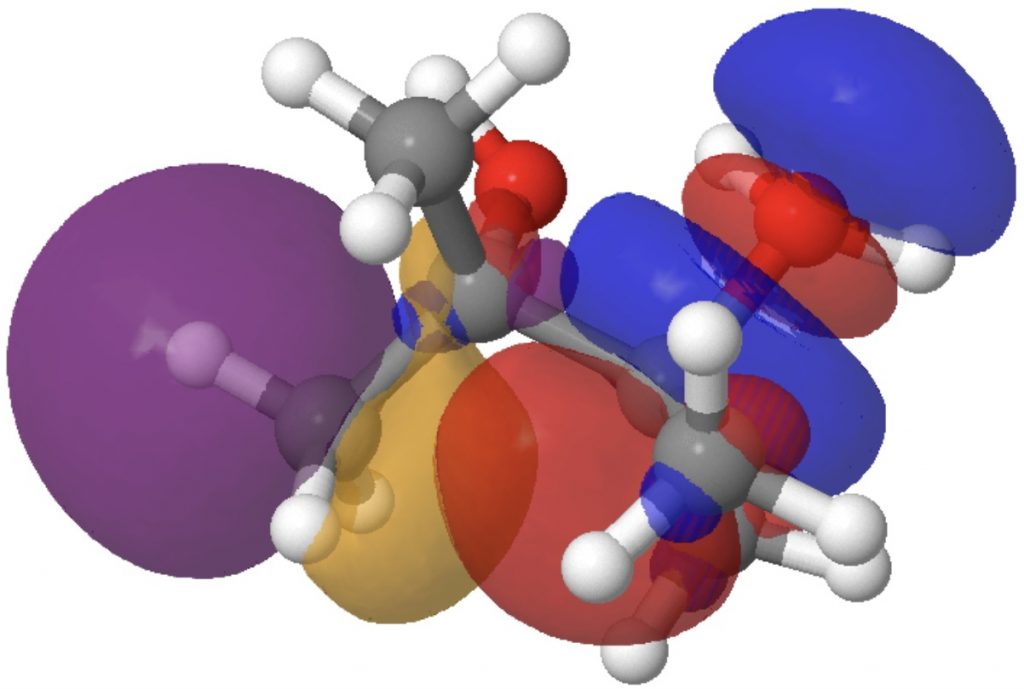
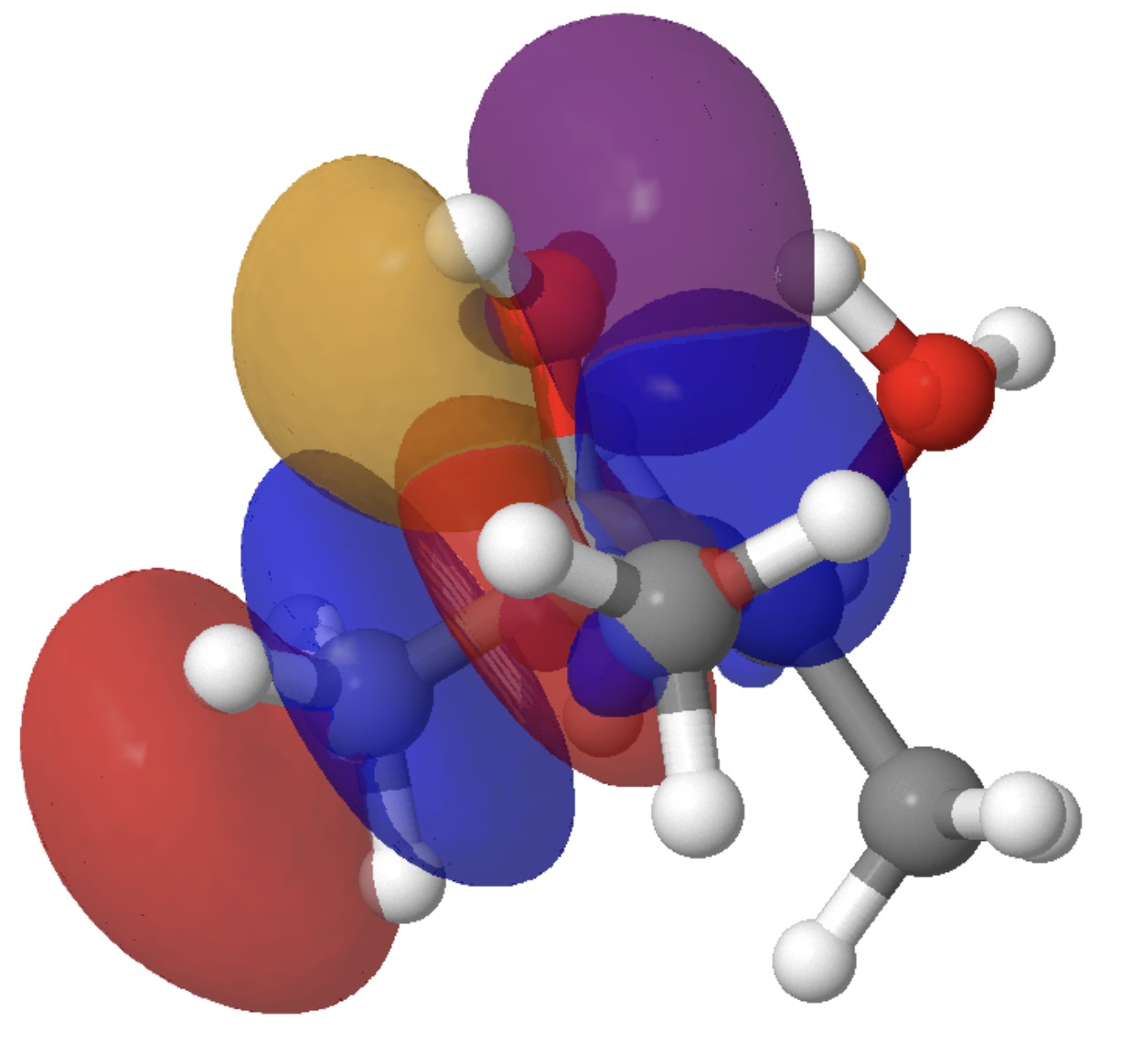
By the point the transition state is reached, these two interactions have developed to the next:
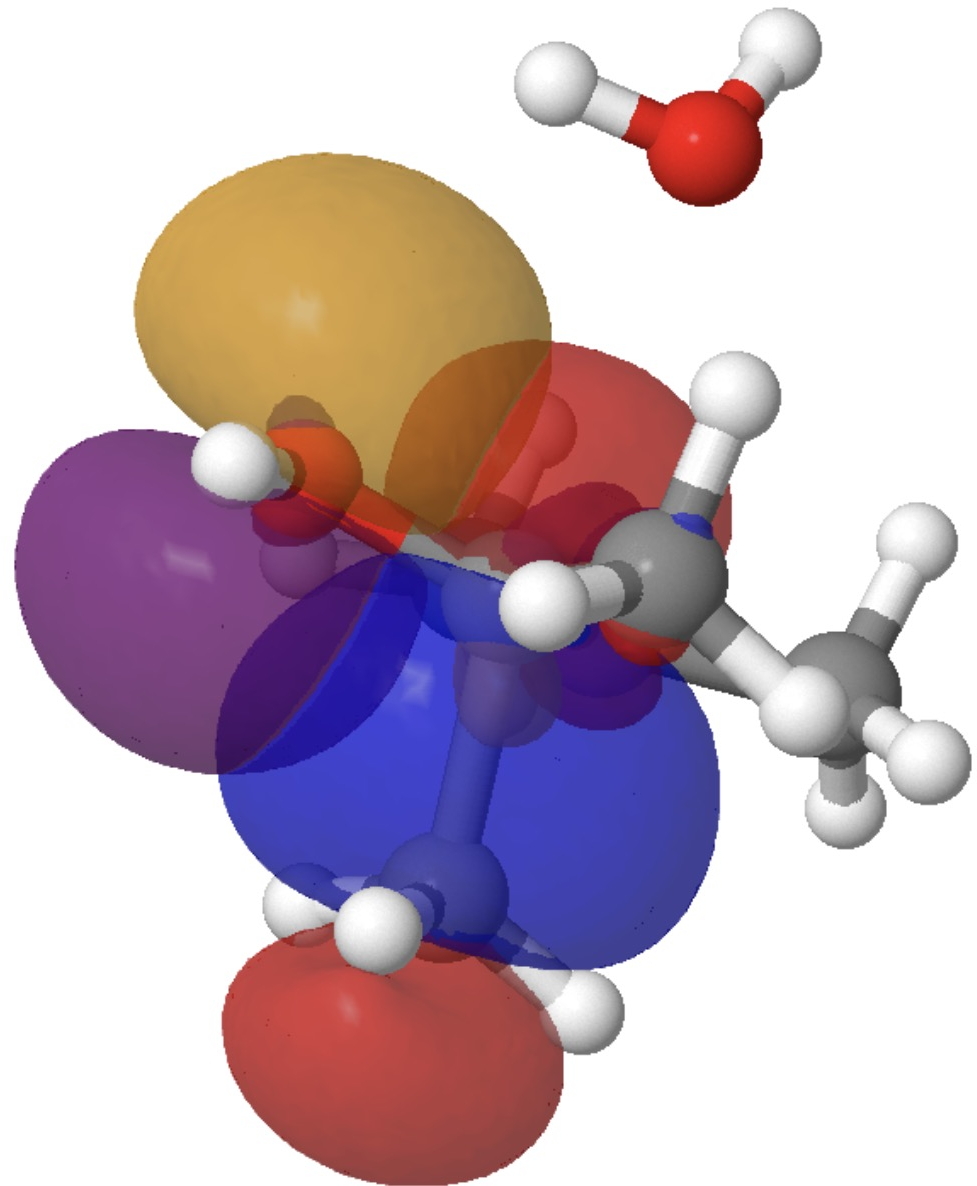
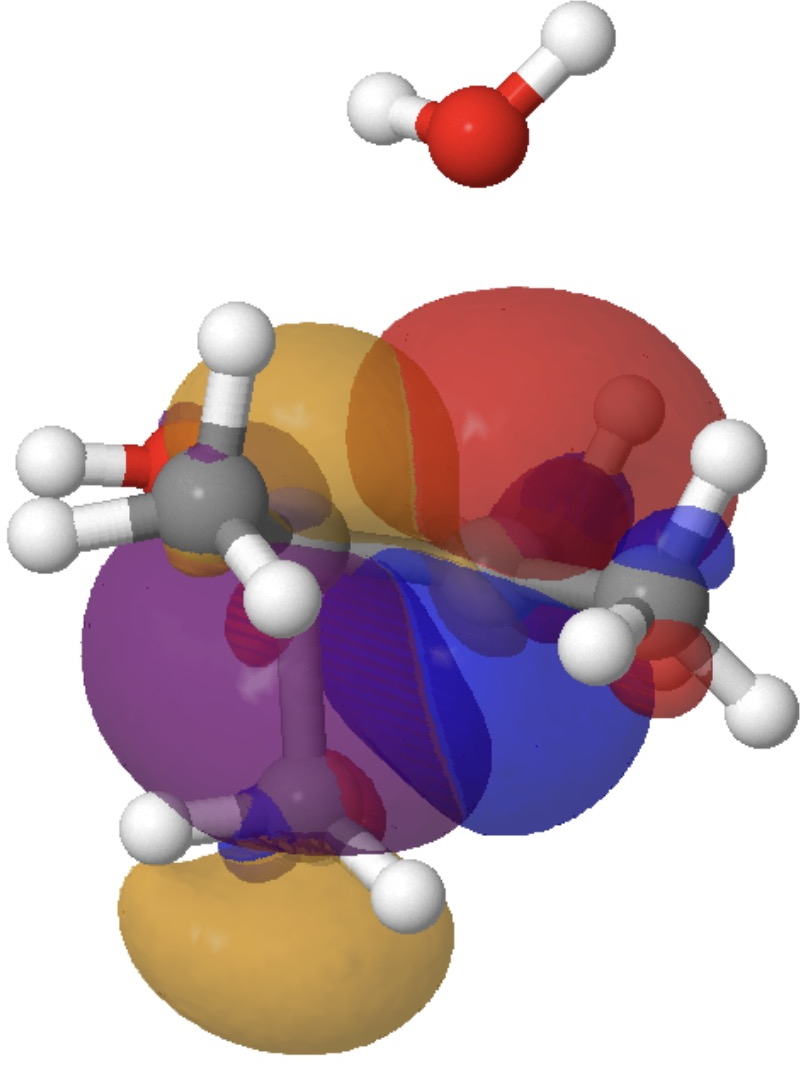
So this venerable response has some good refined stereoelectronic behaviour. These methyl rotations have been disregarded right here, however a deeper look into them may additionally be worthwhile. There may be way more to this response, however I’ll go away this evaluation right here.
This submit has DOI https://doi.org/10.14469/hpc/12684
[ad_2]